Synthesis method of phthalazine derivative
A synthesis method and technology of derivatives, applied in the field of chemistry, can solve the problems of toxic metals, large catalyst loading, cumbersome post-treatment process, etc., and achieve the effects of easy recovery, low cost and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
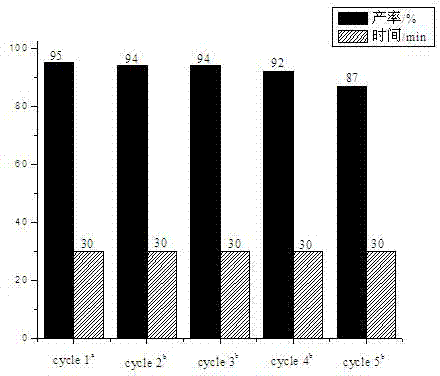
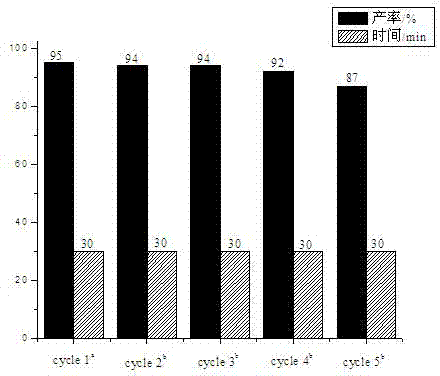
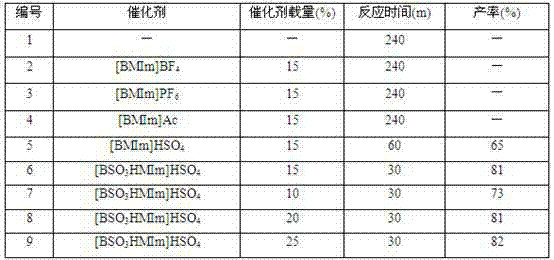
[0022] Example 1, the influence of catalyst type and loading
[0023] Ionic liquids (ILs) have recyclability and good solubility, not only as solvents but also as good catalysts. In this example, the reaction of phthalic hydrazide, dimedone and p-nitrobenzaldehyde was used as a template reaction, and the catalytic activity and loading of the ionic liquid were investigated. The experimental method is as follows: add phthalic hydrazide (1.0 mmol), dimedone (1.0 mmol) and p-nitrobenzaldehyde (1.2 mmol) to the reaction flask, without adding catalyst or adding the following ionic liquids as Catalyst: 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIm]BF 4 ), 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIm]PF 6 ), 1-butyl-3-methylimidazole acetate [BMIm]Ac, 1-butyl-3-methylimidazole hydrogen sulfate ([BMIm]HSO 4 ), 1-butylsulfonic acid-3-methylimidazole hydrogen sulfate ([BSO 3 HMIm]HSO 4 ), then 1 ml of PEG 600 was added, and the reaction temperature was controlled ...
Embodiment 2
[0028] Embodiment 2, the influence of reaction solvent and reaction temperature
[0029] In this example, the reaction of phthalic hydrazide, dimedone and p-nitrobenzaldehyde is used as a template reaction, and [BSO 3 HMIm]HSO 4 As the catalyst, the reaction solvent and reaction temperature were investigated. The experimental method is: add phthalic hydrazide (1.0 mmol), dimedone (1.0 mmol), p-nitrobenzaldehyde (1.2 mmol) and [BSO] into the reaction flask 3 HMIm]HSO 4 (0.15 mmol), then added solvent, and the reaction was carried out under temperature control for 30 minutes. The post-processing method was the same as that in Example 1. The results are shown in Table 2.
[0030] Table 2 Influence of reaction solvent and temperature
[0031]
[0032]It can be seen from Table 2 that (1) using water or ethanol as a solvent, the yield of reflux reaction for 30 minutes is very low, while using PEG 600 as a solvent, the yield of reaction at 120° C. for 30 minutes reaches 81%...
Embodiment 3
[0033] Embodiment 3, the influence of the molar ratio of substrate feeding
[0034] This embodiment takes the reaction of phthalic hydrazide, dimedone and p-nitrobenzaldehyde as a template reaction, and [BSO 3 HMIm] HSO 4 As a catalyst, the molar ratio of substrate to feed was investigated. The experimental method is: add phthalic hydrazide (1.0mmol), dimedone (1.0~1.5mmol), p-nitrobenzaldehyde (1.2~1.7mmol) and [BSO 3 HMIm] HSO 4 (0.15 mmol), and then add 1ml PEG 600, and react at a temperature of 120°C for 30 minutes, and the post-treatment method is the same as in Example 1. The results are shown in Table 3.
[0035] Table 3 The effect of substrate molar ratio
[0036]
[0037] As can be seen from Table 3, along with the increase of the molar ratio of phthalic hydrazide, dimedone and p-nitrobenzaldehyde, the productive rate increases gradually, and when the molar ratio of the three reaches 1:1.4:1.6, the productive rate is the highest Reach 95%, continue to incre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com