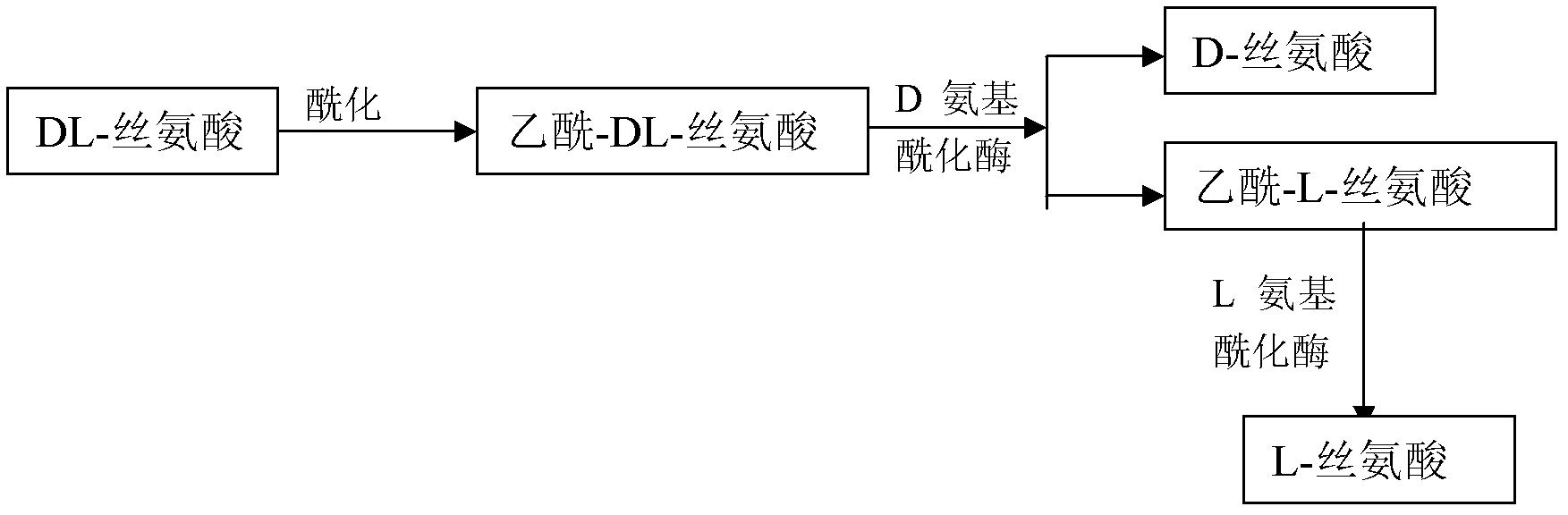
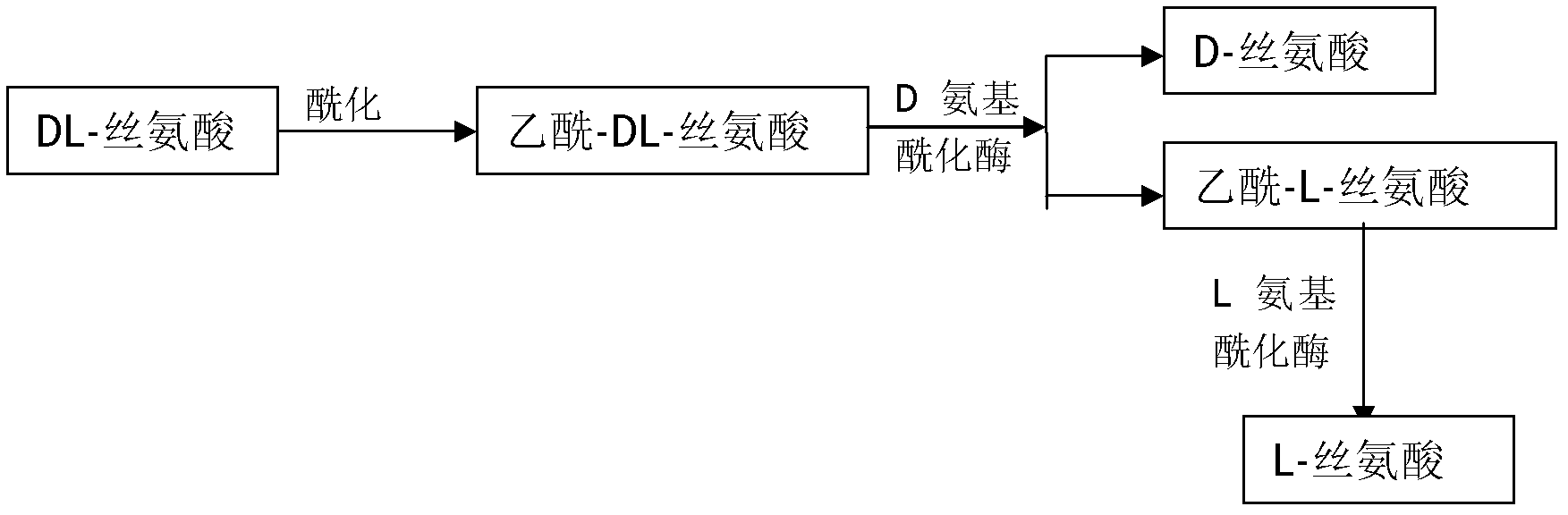
Method for preparing D-serine and L-serine with DL-serine as raw material
A serine and raw material technology, applied in the field of preparation of D-serine and L-serine, can solve the problems of low utilization rate of raw materials, low optical purity, low optical purity of products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1) Acylation in alkaline solution to prepare N-acetyl-DL-serine;
[0017] Take 1000mol of D, L-serine and add to 0.5m 3 In a reaction kettle with purified water, stir and add 215L sodium hydroxide solution dropwise until the serine is completely dissolved, then add 105L acid anhydride dropwise to ensure that the pH value is between 9 and 11, and acylation reaction occurs below 30°C to form N-acetyl-D , L-serine, about 850L of acylation stock solution can be obtained.
[0018] 2) hydrolyzing N-acetyl D-serine in 1) with D-aminoacylase;
[0019] Dilute the acylation stock solution with purified water, and adjust the pH to 7.9-8.2, so that the volume of the split solution is 5m 3 , the concentration is about 0.2mol / L. Add 5g of D-aminoacylase, raise the temperature to 38-39°C, keep it warm for 48 hours, and when the optical rotation of the resolution solution is measured Heat the split solution to 70-80°C, add 1-2 g / L activated carbon, keep it warm for 30 minutes, and...
Embodiment 2
[0029] 1) Acylation in alkaline solution to prepare N-acetyl-DL-serine;
[0030] Take 1000mol of D, L-serine and add to 0.5m 3 In the reaction kettle of purified water, stir, and at the same time, dropwise add 215L sodium hydroxide solution until the serine is completely dissolved. Then add 105L of acid anhydride dropwise to ensure that the pH value is between 9 and 11, and acylation reaction occurs below 30°C to generate N-acetyl-D and L-serine, and about 850L of acylation stock solution can be obtained.
[0031] 2) hydrolyzing N-acetyl D-serine in 1) with D-aminoacylase;
[0032] Dilute the acylation stock solution with purified water, and adjust the pH to 7.9-8.2, so that the volume of the split solution is 5m 3, the concentration is about 0.2mol / L. Add 50g of D-aminoacylase, raise the temperature to 38-39°C, keep it warm for 60 hours, and when the optical rotation of the split solution is measured Heat the split solution to 70-80°C, add 1-2 g / L activated carbon, keep ...
Embodiment 3
[0042] 1) Acylation in alkaline solution to prepare N-acetyl-DL-serine;
[0043] Take 1000mol of D, L-serine and add to 0.5m 3 In the reaction kettle of purified water, stir, and at the same time, dropwise add 215L sodium hydroxide solution until the serine is completely dissolved. Then add 105L of acid anhydride dropwise to ensure that the pH value is between 9 and 11, and acylation reaction occurs below 30°C to generate N-acetyl-D and L-serine, and about 850L of acylation stock solution can be obtained.
[0044] 2) hydrolyzing N-acetyl D-serine in 1) with D-aminoacylase;
[0045] Dilute the acylation stock solution with purified water, and adjust the pH to 7.9-8.2, so that the volume of the split solution is 5m 3 , the concentration is about 0.2mol / L. Add 90g of D-aminoacylase, raise the temperature to 38-39°C, keep it warm for 72 hours, and when the optical rotation of the resolution solution is measured Heat the split solution to 70-80°C, add 1-2 g / L activated carbon,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com