Controlled-release injection containing antidiuresis components and preparation method thereof
A slow-release injection and anti-diuretic technology, applied in the field of preparations, can solve problems such as inconvenient clinical use and medication for patients, easy entry of viruses and bacteria into mucous membranes, and frequent administration of injections.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 20mg main drug desmopressin acetate, 80mg PLA, special solvent is 1.5% sodium carboxymethyl cellulose normal saline.
[0020] Its preparation process is as follows:
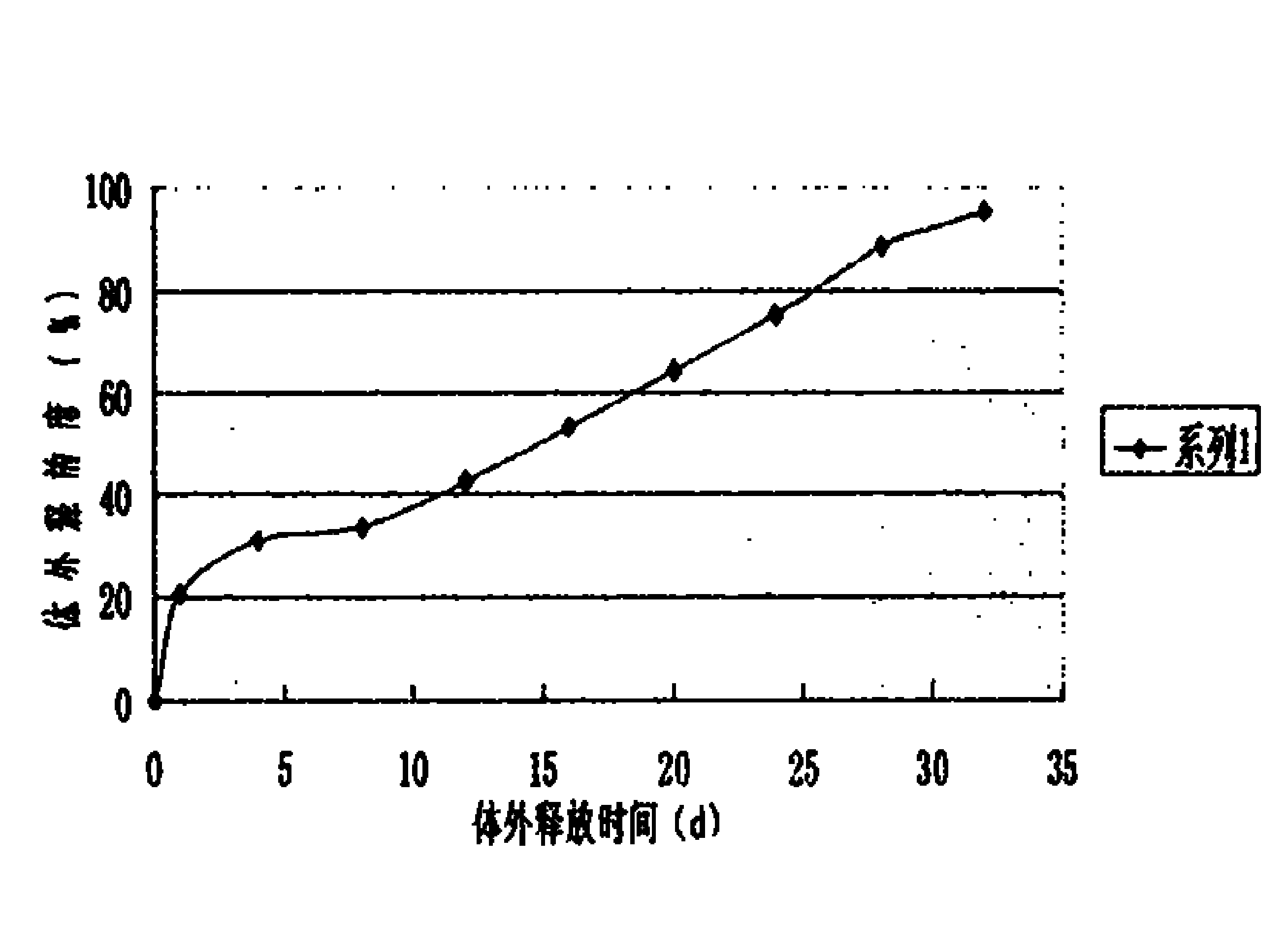
[0021] Put 80mg of polylactic acid (PLA) into a container, add 100ml of dichloromethane, dissolve and mix well, add 20mg of desmopressin acetate, reshake well, and then vacuum dry to remove the organic solvent. The dried drug-containing solid composition is frozen and pulverized to make sustained-release microparticles containing 20% desmopressin acetate, and then suspended in 5000ml of physiological saline containing 1.5% (percentage by weight) sodium carboxymethylcellulose to prepare Get the corresponding suspension type sustained-release injection.
[0022] 5000 bottles of desmopressin acetate sustained-release injections were prepared in this example, and each bottle contained 4 μg of the main drug (1 ml / bottle). Through the determination of the release degree, the sustained-release effect and clin...
Embodiment 2
[0028] 20mg main drug desmopressin acetate, 80mg PLA, special solvent is 1.5% sodium carboxymethylcellulose and 0.1% Tween 80 normal saline. Its preparation process is as follows:
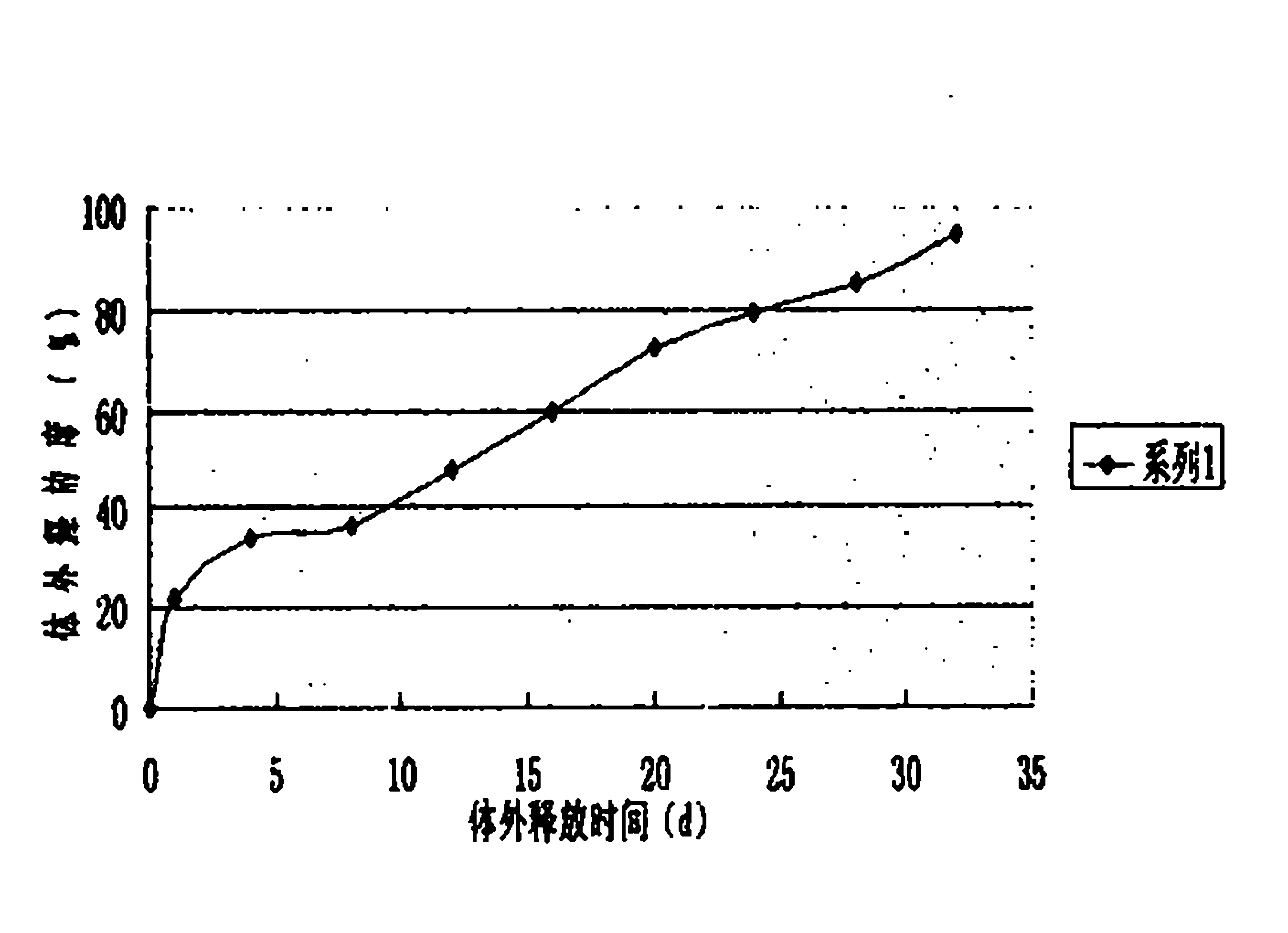
[0029] Put 80mg of polylactic acid (PLA) into a container, add 100ml of dichloromethane, dissolve and mix well, add 20mg of desmopressin acetate, reshake well, and then vacuum dry to remove the organic solvent. The dried drug-containing solid composition is frozen and pulverized to make slow-release microparticles containing 20% desmopressin acetate, and then suspended in 5000ml containing 1.5% (weight percent) sodium carboxymethylcellulose and 0.1% Tween 80 The corresponding suspension-type sustained-release injection was prepared in normal saline.
[0030] 5000 bottles of desmopressin acetate sustained-release injections were prepared in this example, and each bottle contained 4 μg of the main drug (1 ml / bottle). Through the determination of the release degree, the sustained-release effect an...
Embodiment 3
[0036] 20mg main drug desmopressin acetate, 80mg PLA, the special solvent is 1.5% sodium carboxymethylcellulose, 15% sorbitol and 0.1% Tween 80 normal saline. Its preparation process is as follows:
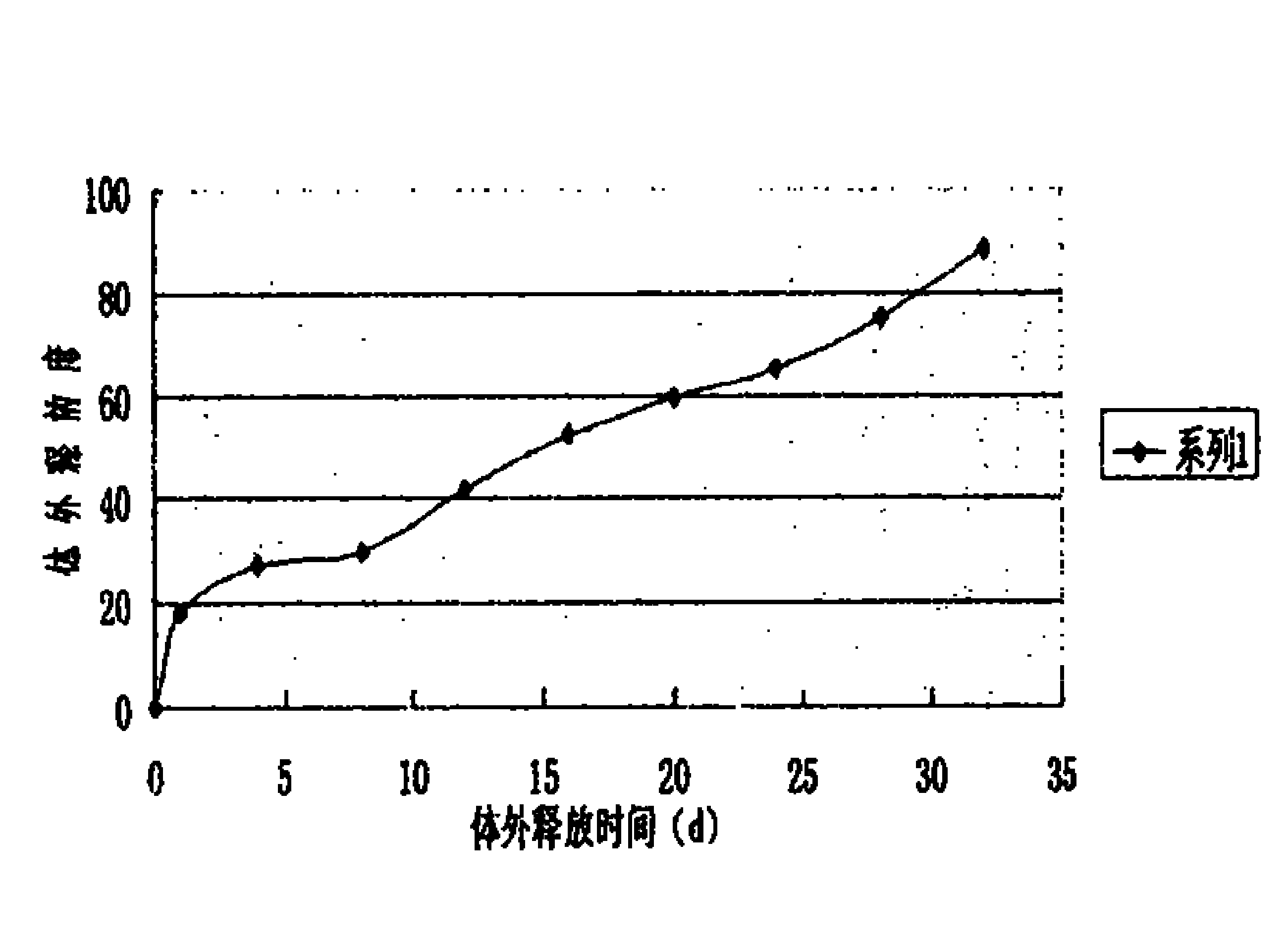
[0037] Put 80mg of polylactic acid (PLA) into a container, add 100ml of dichloromethane, dissolve and mix well, add 20mg of desmopressin acetate, reshake well, and then vacuum dry to remove the organic solvent. The dried drug-containing solid composition is frozen and pulverized to make slow-release microparticles containing 20% desmopressin acetate, and then suspended in 5000ml containing 1.5% (percentage by weight) sodium carboxymethylcellulose and 15% sorbitol and 0.1 % Tween 80 in normal saline to prepare the corresponding suspension-type sustained-release injection.
[0038] 5000 bottles of desmopressin acetate sustained-release injections were prepared in this example, and each bottle contained 4 μg of the main drug (1 ml / bottle). Through the determination of the release d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com