Aminomethylation method for tocopherol concentrated solution
A technology of amine methylation and tocopherol, which is applied in the field of amine methylation of tocopherol concentrate, can solve the problems of complex process, hindering production progress, low yield, etc., and achieve stable products, stable production technology, The effect of mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
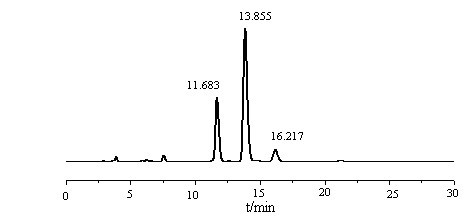
[0030] All instruments and equipment are laboratory-scale. The content of mixed tocopherols in the raw tocopherol concentrate is 36.30%, of which α-tocopherol accounts for 8.09%, β, γ-tocopherol accounts for 63.03%, and δ-tocopherol accounts for 28.88%.
[0031] Take 75g of tocopherol concentrate (about 0.06mol of non-α-tocopherol) in the reactor, add 21.6g (about 0.19mol) of dimethylamine aqueous solution (40%), then add 106mL of toluene solvent, and open it after purging three times with nitrogen Stir, control the temperature below 35°C, slowly add 16.5g (about 0.20mol) formaldehyde aqueous solution (37%) into the reactor dropwise within 20min, then raise the temperature to 90°C, condense and reflux for 2.5h to stop the reaction; Liquid separator, after standing for 0.5h, drain the lower water phase and the impurities in the interlayer, transfer the mother liquor to the desolventizer; finally raise the temperature to 120°C to distill the toluene out, stop the reaction when th...
Embodiment 2
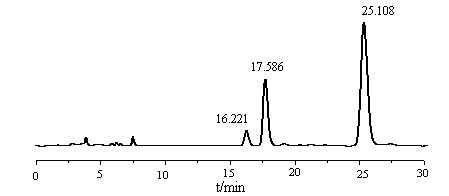
[0036] Take 75g of tocopherol concentrate (about 0.06mol of non-α-tocopherol) in the reactor, add 21.6g (about 0.19mol) of dimethylamine aqueous solution (40%), then add 106ml of cyclohexane solvent, and blow nitrogen three times Then start stirring, control the temperature below 35°C, slowly add 16.5g (about 0.20mol) formaldehyde aqueous solution (37%) into the reactor dropwise within 20min, then raise the temperature to 80°C, condense and reflux for 2.5h and then stop the reaction; The liquid was transferred into the liquid separator, and after standing for 0.5h, the impurities in the lower aqueous phase and the interlayer were discharged, and the mother liquor was transferred to the desolventizer; finally, the temperature was raised to 90°C to distill the cyclohexane, and the reaction was stopped when there was no distillate. , cooling, sampling and weighing, the product is a mixture of impurities other than α-tocopheryl alkaloids and glycerides.
[0037] Take 2-3 drops of ...
Embodiment 3
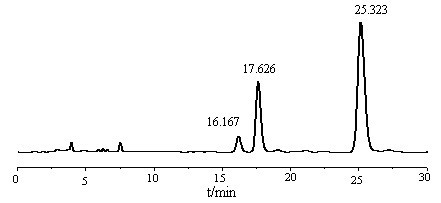
[0041] Take 75g of tocopherol concentrate (about 0.06mol of non-α-tocopherol) in the reactor, add 36.5g (about 0.20mol) of diethylamine aqueous solution (40%), then add 110ml of cyclohexane solvent, and blow nitrogen three times Then start stirring, control the temperature below 35°C, slowly add 16.5g (about 0.20mol) formaldehyde aqueous solution (37%) into the reactor dropwise within 20min, then raise the temperature to 85°C, condense and reflux for 4.5h to stop the reaction; The liquid was transferred into the liquid separator, and after standing for 0.5h, the impurities in the lower aqueous phase and the interlayer were discharged, and the mother liquor was transferred to the desolventizer; finally, the temperature was raised to 90°C to distill the cyclohexane, and the reaction was stopped when there was no distillate. , cooling, sampling and weighing, the product is a mixture of impurities other than α-tocopheryl alkaloids and glycerides.
[0042] Take 2-3 drops of the pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com