Solid electrolyte for solar cell based on ionic crystal
A solid electrolyte and solar cell technology, applied in the field of dye-sensitized solar cells, can solve problems such as unsatisfactory cell conversion efficiency, and achieve the effects of increasing light scattering, high photoelectric conversion efficiency, and wide applicable temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
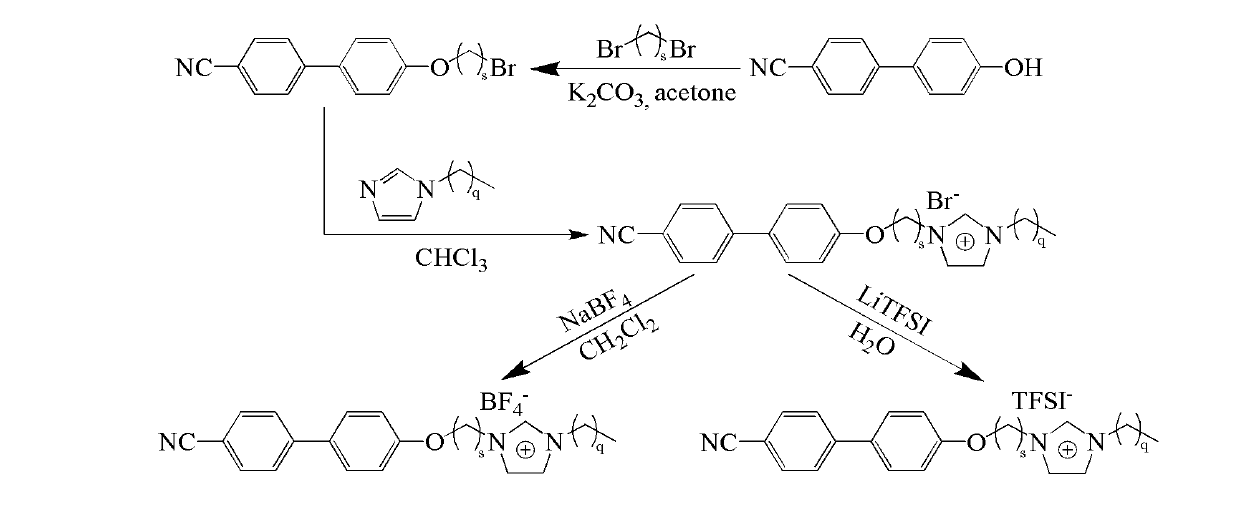
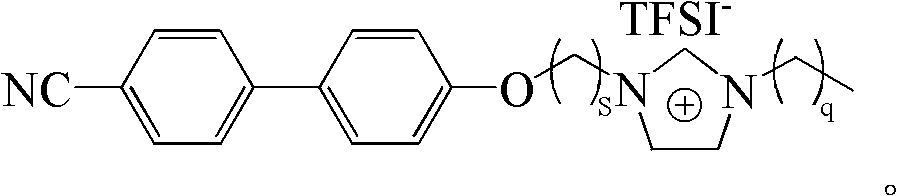
[0025] See figure 1 As shown, ([C 2 Synthesis of MIm][Br]): refer to the literature (Synthesis 2008, 2008, 2551-2560; J. Mater. Chem. 2011, 21, 7326-7330), 15mmol of dibromoethane and 20mmol of K 2 CO 3 Add to 10mmol Acetone (20mL) solution, N 2 Under protection, react at 70°C for 12h. Filter and remove the solvent to obtain a precipitate, which is recrystallized from petroleum ether to obtain 1 HNMR(400MHz, CDCl 3 ): 7.67 (m, 4H), 7.54 (d, 2H), 7.02 (d, 2H), 4.35 (t, 2H), 3.67 (t, 2H). Take 25mmol KOH and add to 10mmol In a chloroform (20 mL) solution mixed with 12 mmol of methylimidazole, the reaction was carried out in an ice-water bath for 1 hour, and then at 45°C for 48 hours. The solvent was removed by rotary evaporation, and a white solid was obtained after washing three times with ether and ethyl acetate. 1 HNMR(300MHz, CDCl 3 ): 10.50 (s, 1H), 7.63 (m, 4H), 7.51 (d, 2H), 7.28 (d, 2H), 7.01 (d, 2H), 4.94 (m, 2H), 4.49 (m, 2H) , 4.08 (s, 3H).
Embodiment 2
[0027] See figure 1 As shown, ([C 2 EIm][Br]): refer to the literature (Synthesis 2008, 2008, 2551-2560; J. Mater. Chem. 2011, 21, 7326-7330), 15mmol of dibromoethane and 20mmol K 2 CO 3 Add to 10mmol Acetone (20mL) solution, N 2 Under protection, react at 70°C for 12h. Filter and remove the solvent to obtain a precipitate, which is recrystallized from petroleum ether to obtain 1 HNMR(400MHz, CDCl 3 ): 7.67 (m, 4H), 7.54 (d, 2H), 7.02 (d, 2H), 4.35 (t, 2H), 3.67 (t, 2H). Take 25mmol KOH and add to 10mmol In a chloroform (20 mL) solution mixed with 12 mmol of ethyl imidazole, react for 1 h in an ice-water bath, and then at 45° C. for 48 h. The solvent was removed by rotary evaporation, and a white solid was obtained after washing three times with ether and ethyl acetate. 1 HNMR(300MHz, CDCl 3 ): 10.49(s, 1H), 7.64(m, 4H), 7.51(d, 2H), 7.30(d, 2H), 7.00(d, 2H), 4.38(t, 2H), 3.66(s, 2H) , 2.64(t, 2H), 1.02(s, 3H).
Embodiment 3
[0029] See figure 1 As shown, ([C 2 BIm][Br]) synthesis: refer to literature (Synthesis 2008, 2008, 2551-2560; J. Mater. Chem. 2011, 21, 7326-7330), 15mmol of dibromoethane and 20mmol K 2 CO 3 Add to 10mmol Acetone (20mL) solution, N 2 Under protection, react at 70°C for 12h. Filter and remove the solvent to obtain a precipitate, which is recrystallized from petroleum ether to obtain 1 HNMR(400MHz, CDCl 3 ): 7.67 (m, 4H), 7.54 (d, 2H), 7.02 (d, 2H), 4.35 (t, 2H), 3.67 (t, 2H). Take 25mmol KOH and add to 10mmol In a chloroform (20 mL) solution mixed with 12 mmol of butyl imidazole, react for 1 hour in an ice-water bath, and then react at 45°C for 48 hours. The solvent was removed by rotary evaporation, and a white solid was obtained after washing three times with ether and ethyl acetate. 1 HNMR(300MHz, CDCl 3 ): 10.47 (s, 1H), 7.64 (m, 4H), 7.50 (d, 2H), 7.39 (d, 2H), 6.97 (d, 2H), 4.48 (t, 2H), 4.09 (s, 3H) , 2.18(t, 2H), 1.92(s, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com