Solid electrolyte for solar cell based on ionic crystal
A solid-state electrolyte and solar cell technology, applied in the field of dye-sensitized solar cells, can solve the problems of unsatisfactory conversion efficiency of cells, and achieve the effects of increasing light scattering, wide applicable temperature range, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
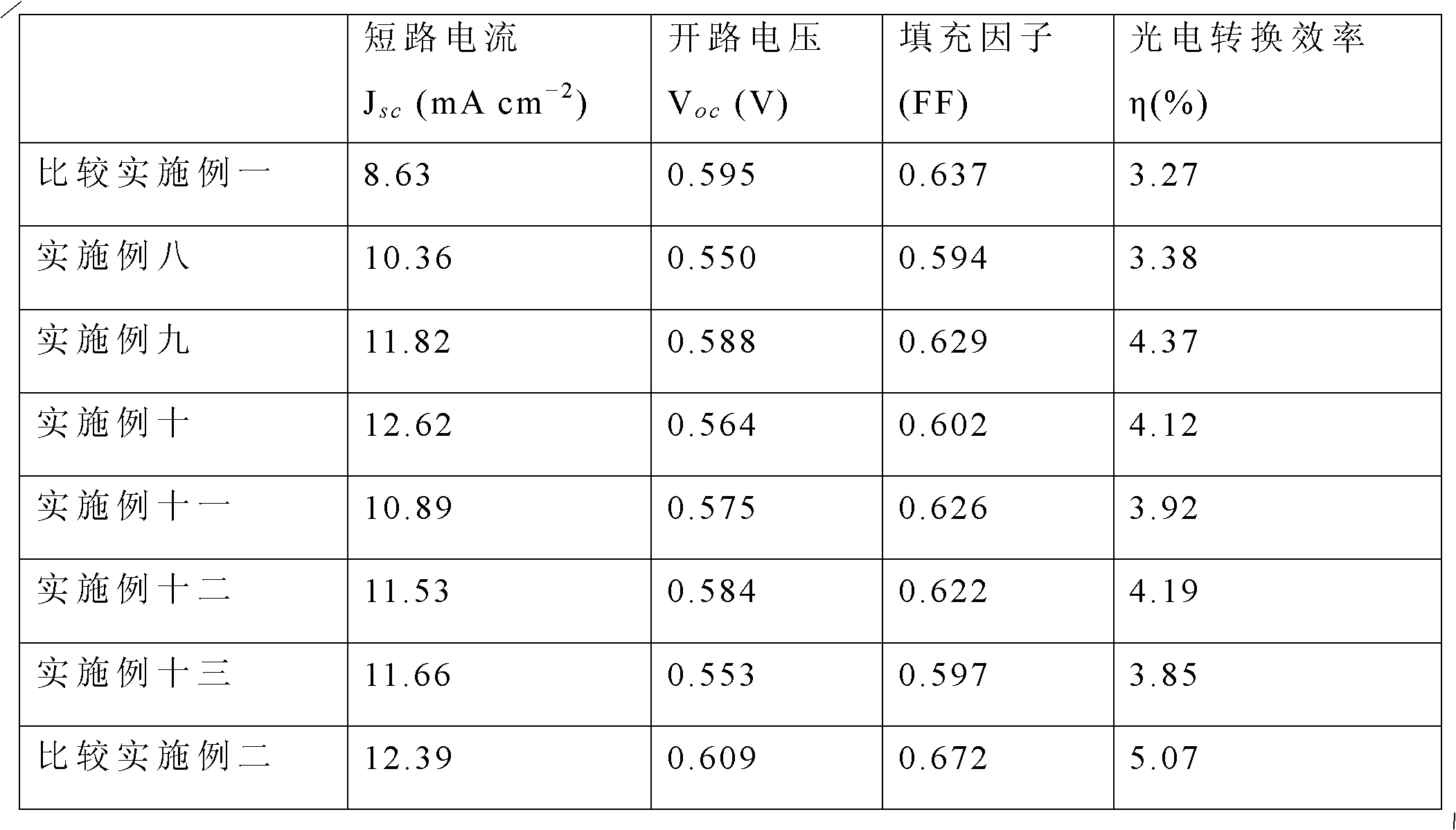
Examples
Embodiment 1
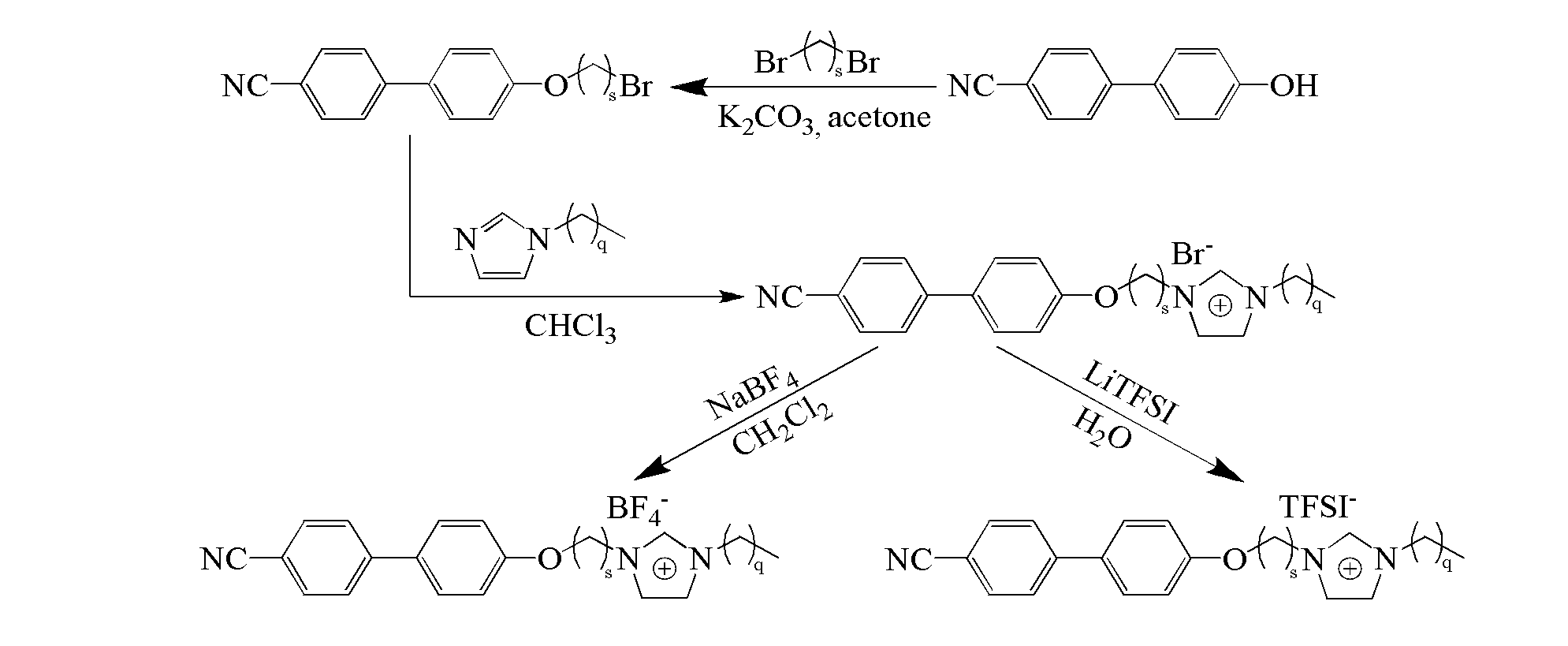
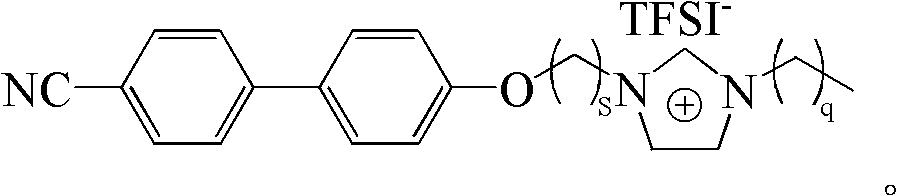
[0025] see figure 1 as shown, ([C 2 MIm][Br]) synthesis: referring to literature (Synthesis 2008, 2008, 2551-2560; J.Mater.Chem.2011, 21, 7326-7330), 15mmol dibromoethane and 20mmol K 2 CO 3 were added to 10mmol In acetone (20mL) solution, N 2 Under protection, react at 70°C for 12h. Filtration, and removal of the solvent resulted in a precipitate, which was recrystallized from petroleum ether to obtain 1 HNMR (400MHz, CDCl 3 ): 7.67 (m, 4H), 7.54 (d, 2H), 7.02 (d, 2H), 4.35 (t, 2H), 3.67 (t, 2H). Take 25mmol KOH and add to 10mmol In a chloroform (20 mL) solution mixed with 12 mmol of methylimidazole, react in an ice-water bath for 1 h, and then react at 45° C. for 48 h. The solvent was removed by rotary evaporation, and a white solid was obtained after washing three times with ether and ethyl acetate respectively. 1 HNMR (300MHz, CDCl 3 ): 10.50(s, 1H), 7.63(m, 4H), 7.51(d, 2H), 7.28(d, 2H), 7.01(d, 2H), 4.94(m, 2H), 4.49(m, 2H) , 4.08(s, 3H).
Embodiment 2
[0027] see figure 1 as shown, ([C 2 EIm][Br]) synthesis: with reference to literature (Synthesis 2008, 2008, 2551-2560; J.Mater.Chem.2011, 21, 7326-7330), 15mmol dibromoethane and 20mmol K 2 CO 3 were added to 10mmol In acetone (20mL) solution, N 2 Under protection, react at 70°C for 12h. Filtration, and removal of the solvent resulted in a precipitate, which was recrystallized from petroleum ether to obtain 1 HNMR (400MHz, CDCl 3 ): 7.67 (m, 4H), 7.54 (d, 2H), 7.02 (d, 2H), 4.35 (t, 2H), 3.67 (t, 2H). Take 25mmol KOH and add to 10mmol In a chloroform (20 mL) solution mixed with 12 mmol ethylimidazole, react in an ice-water bath for 1 h, and then react at 45° C. for 48 h. The solvent was removed by rotary evaporation, and a white solid was obtained after washing three times with ether and ethyl acetate respectively. 1 HNMR (300MHz, CDCl 3 ): 10.49(s, 1H), 7.64(m, 4H), 7.51(d, 2H), 7.30(d, 2H), 7.00(d, 2H), 4.38(t, 2H), 3.66(s, 2H) , 2.64(t, 2H), 1.02(s, 3H)....
Embodiment 3
[0029] see figure 1 as shown, ([C 2 BIm][Br]) Synthesis: with reference to literature (Synthesis 2008, 2008, 2551-2560; J.Mater.Chem.2011, 21, 7326-7330), 15mmol dibromoethane and 20mmol K 2 CO 3 were added to 10mmol In acetone (20mL) solution, N 2 Under protection, react at 70°C for 12h. Filtration, and removal of the solvent resulted in a precipitate, which was recrystallized from petroleum ether to obtain 1 HNMR (400MHz, CDCl 3): 7.67 (m, 4H), 7.54 (d, 2H), 7.02 (d, 2H), 4.35 (t, 2H), 3.67 (t, 2H). Take 25mmol KOH and add to 10mmol In a chloroform (20 mL) solution mixed with 12 mmol butylimidazole, react in an ice-water bath for 1 h, and then react at 45° C. for 48 h. The solvent was removed by rotary evaporation, and a white solid was obtained after washing three times with ether and ethyl acetate respectively. 1 HNMR (300MHz, CDCl 3 ): 10.47(s, 1H), 7.64(m, 4H), 7.50(d, 2H), 7.39(d, 2H), 6.97(d, 2H), 4.48(t, 2H), 4.09(s, 3H) , 2.18(t, 2H), 1.92(s, 4H). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com