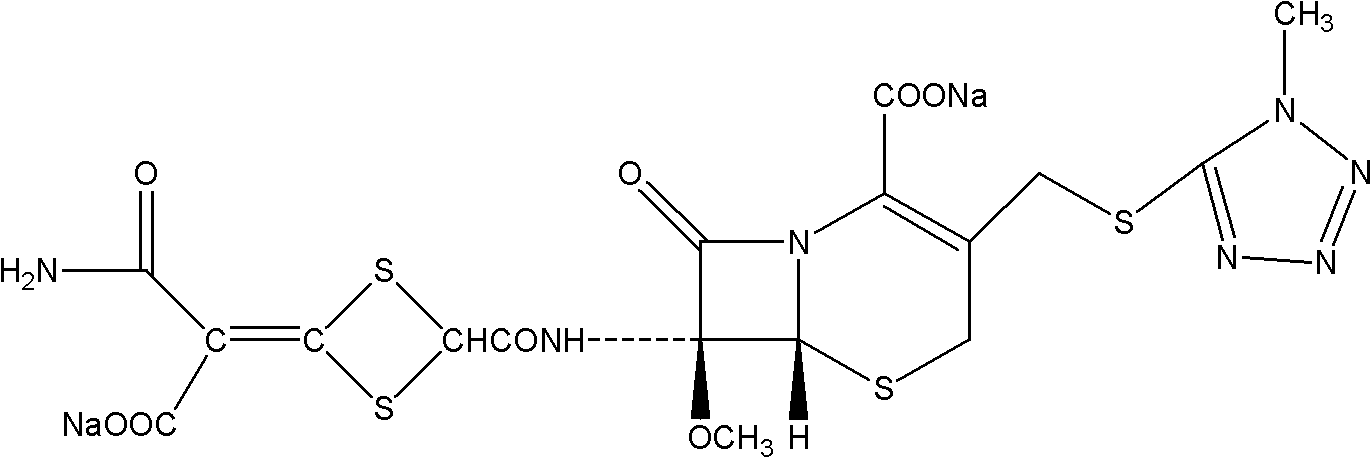
Composition of cefotetan acid and sodium citrate
A technology of cefotetan acid and sodium citrate, which is applied in the direction of drug combination, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of high side effects and allergic reactions, ineffective removal of impurities, Secondary injury of patients and other problems, to achieve the effect of low side reaction and allergy rate, excellent water solubility, and improve product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of powder injection from the composition of cefotetan acid and sodium citrate: in the aseptic ingredient workshop, weigh 50 kg of cefotetan acid and sodium citrate aseptic raw materials according to the proportioning scheme in Table 1, and pour them into the high-efficiency three-dimensional In the motion mixer, set the rotation speed of the three-dimensional powder mixer to 5 rpm, start the powder mixing operation according to the powder mixing packaging standard operating procedures, mix for 60 minutes to 90 minutes until the mixture is uniform, discharge, and pack to obtain powder injections.
[0020] The samples of each ratio were taken to detect the pH value of each sample, and then water for injection was used to prepare a liquid medicine with a concentration for routine infusion of the human body, and the water solubility of each sample solution was tested. The specific experimental results are shown in Table 1.
[0021] The pH value and water-soluble ...
Embodiment 2
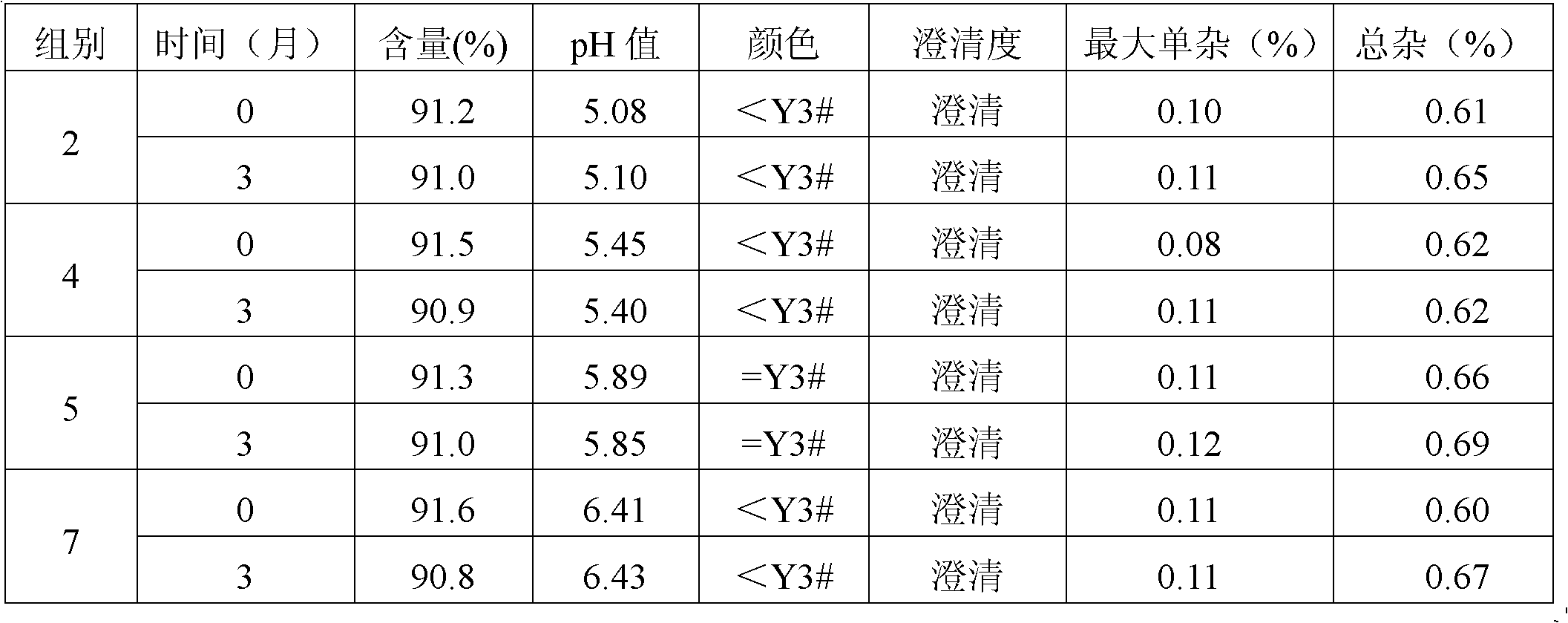
[0024] Example 2 Stability Study of Cefotetan Acid and Sodium Citrate Composition
[0025] According to the long-term test (3 months) and the accelerated test (3 months) test operation standard of the drug registration standard, the stability of the cefotetan acid and sodium citrate compositions of the above groups of 2, 4, 5 and 7 is detected , and compared with single cefotetan disodium. Wherein cefotetan acid and the related substance that is transformed into by cefotetan acid adopt high performance liquid chromatography (HPLC) to detect, use waters2487 type high performance liquid chromatography, with octadecylsilane bonded silica gel as packing column; Acetonitrile-methanol-0.1mol / l potassium dihydrogen phosphate-glacial acetic acid (105:105:1200:100) is the mobile phase; the detection wavelength is 254nm. The number of theoretical plates calculated based on the peak of cefotetan disodium should not be less than 2000. The specific experimental results are shown in Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com