Vanadium dioxide powders and preparation method thereof
A technology for vanadium dioxide and powder, applied in the field of vanadium dioxide powder and its preparation, can solve the problems of high toxicity, low solubility, high price and the like, and achieve the effects of low toxicity, simple preparation method and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
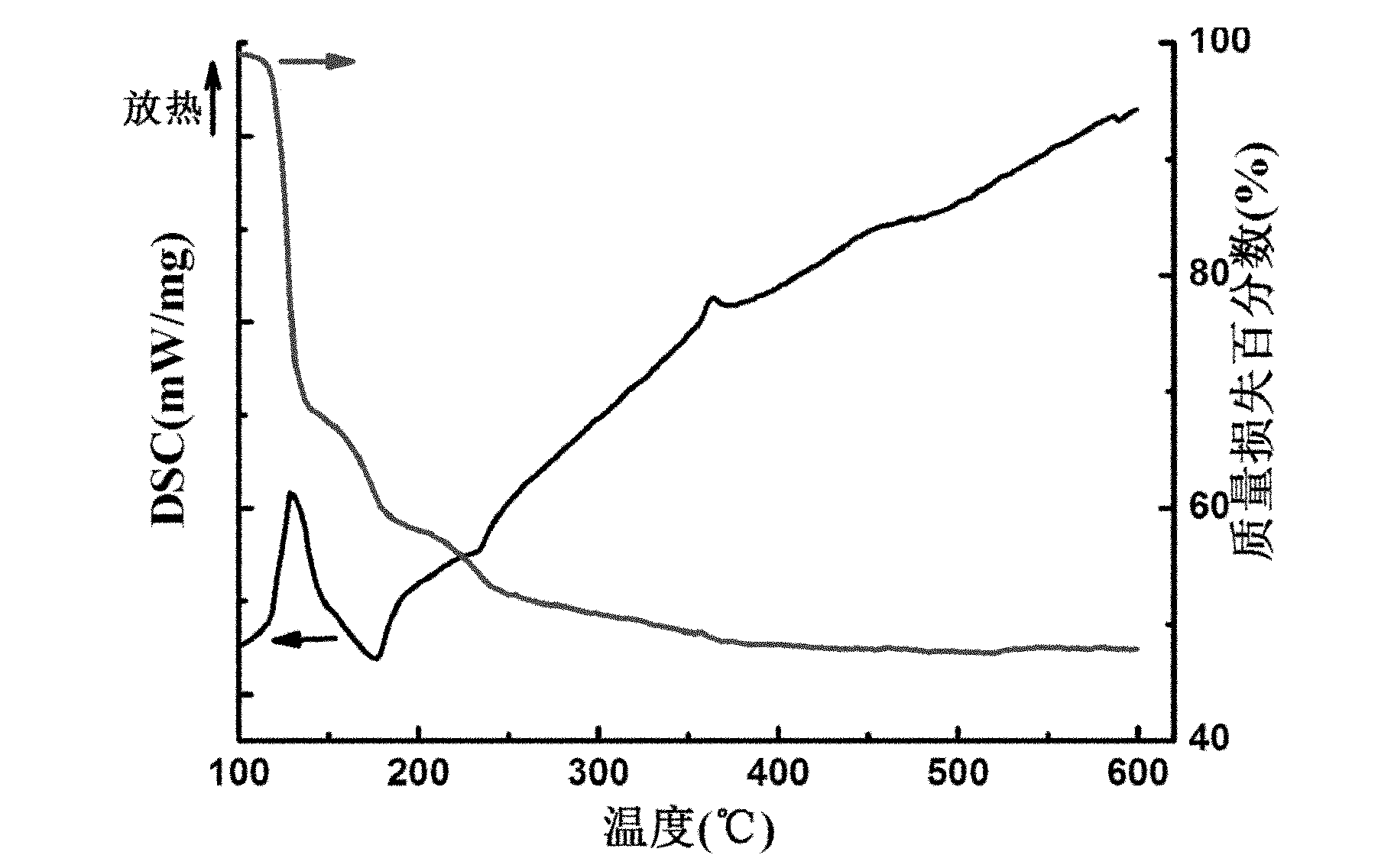
[0035] Add 2.10 grams of citric acid to 50 milliliters of hydrogen peroxide with a mass fraction of 30%, adjust the pH value to 10 with ammonia water, place the above solution in an ice-water bath, slowly add 1.63 grams of vanadyl sulfate, stir for 2 hours, add 50 Milliliter of ethanol and let it stand for 4 hours, the resulting precipitate was separated by filtration to obtain the peroxo complex of vanadium.
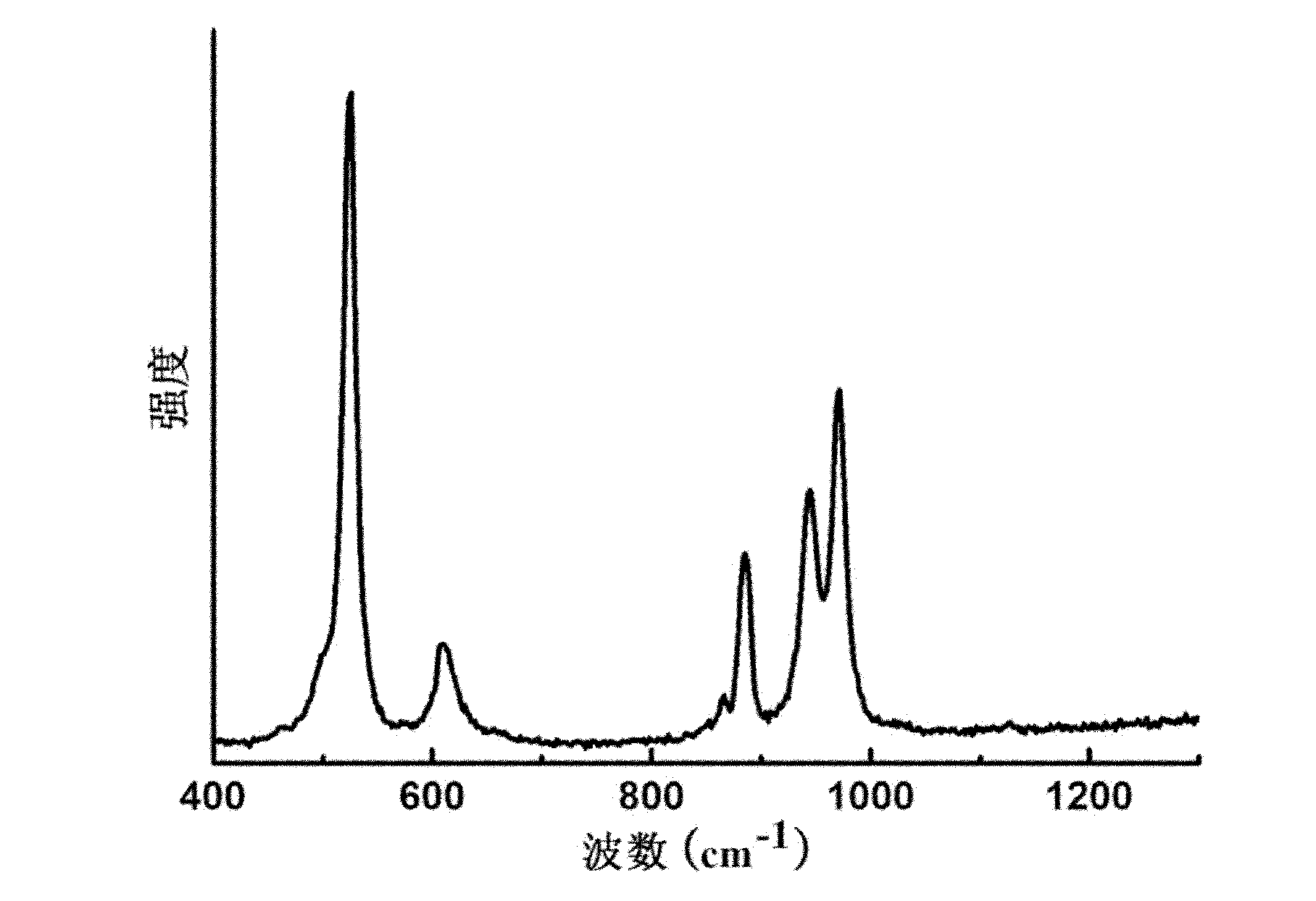
[0036] The Raman spectrum of the product obtained in this embodiment is as follows figure 1 Shown: at 885cm -1 、608cm -1 、526cm -1 The peak at is the characteristic peak of peroxy group, shows that contains peroxy group in the product. 971cm -1 The peak at is the characteristic peak of the V=O bond.
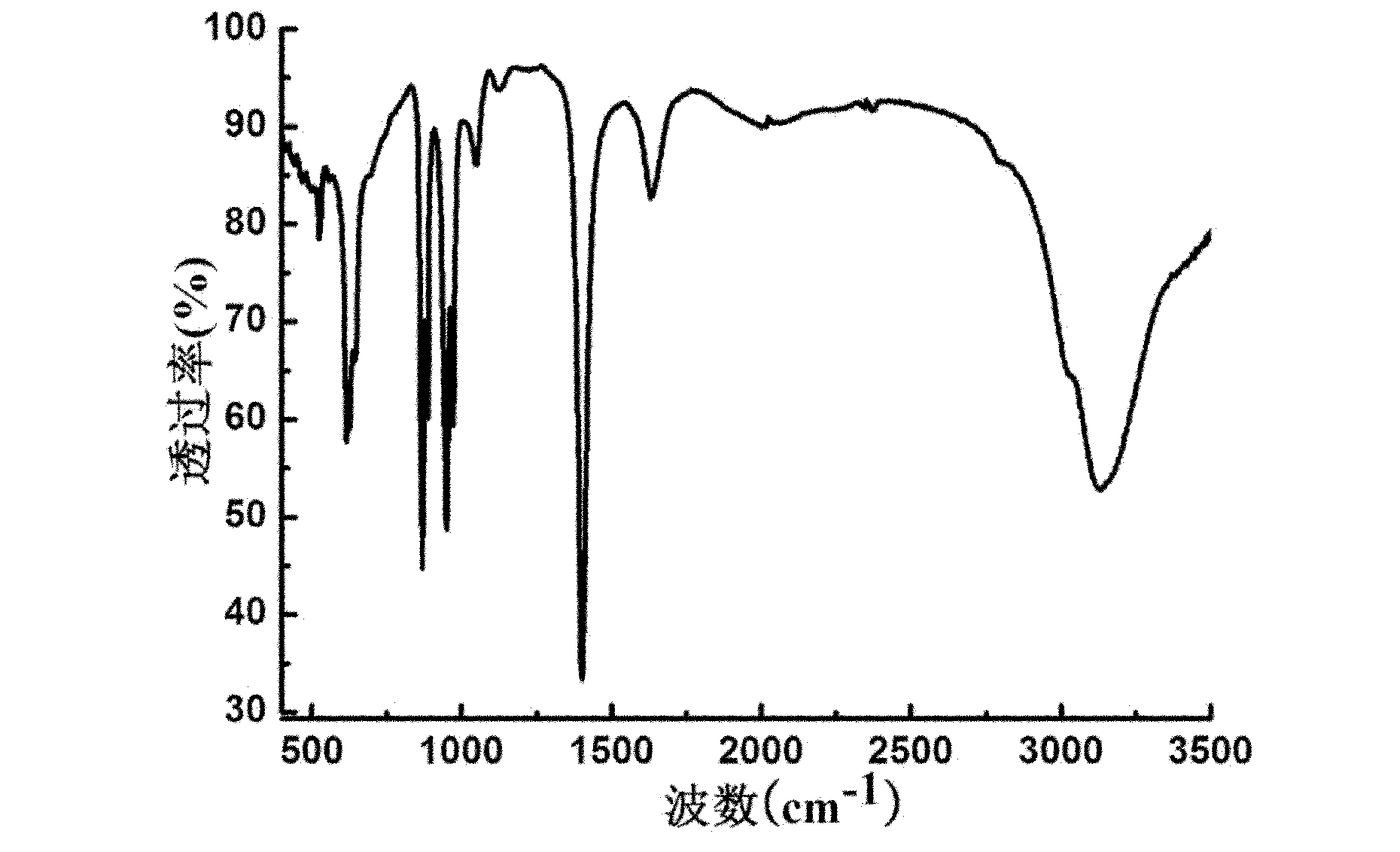
[0037] The infrared spectrum of the product obtained in this embodiment is as follows figure 2 Shown: at 870cm -1 、610cm -1 、523cm -1 The peak at is the characteristic peak of the peroxy group, 950cm -1 The peak at is the characteristic peak of the V=O bond.
...
Embodiment 2
[0045] Dissolve 20 ml of hydrogen peroxide with a mass fraction of 30% in 80 ml of water, add 1.08 g of tartaric acid, adjust the pH value to 12 with ammonia water, place the above solution in an ice-water bath, slowly add 0.78 g of vanadyl sulfate, and stir After 4 hours, 120 ml of isopropanol was added and allowed to stand for 8 hours, and the resulting precipitate was separated by filtration to obtain a vanadium peroxy complex.
[0046] Using Raman and infrared spectroscopy to analyze the functional groups contained in the product obtained in this example, the results are similar to those in Example 1, indicating that it contains peroxy groups.
Embodiment 3
[0048] In 50 milliliters of hydrogen peroxide with a mass fraction of 30%, add 2.40 grams of malic acid, adjust the pH value to 14 with ammonia water, place the above solution in an ice-water bath, slowly add 1.96 grams of vanadyl sulfate, and stir for 4 hours. After adding 80 milliliters of acetone, the mixture was allowed to stand for 6 hours, and the resulting precipitate was separated by filtration to obtain a peroxo complex of vanadium.
[0049] Using Raman and infrared spectroscopy to analyze the functional groups contained in the product obtained in this example, the results are similar to those in Example 1, indicating that it contains peroxy groups.
[0050] The obtained product of embodiment 2 and 3 is through elemental analysis, and its obtained product composition meets general formula V 2 N 3±x h 14±y o 11±z , where the values of x, y, and z are all between 0-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com