Preparation method for etoposide intermediate
A technology for etoposide and intermediates, which is applied in the field of preparation of etoposide intermediates, can solve the problems of complex product post-processing, high fire protection requirements, and high impurity content, and achieve reduced post-reaction processing, high safety, and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
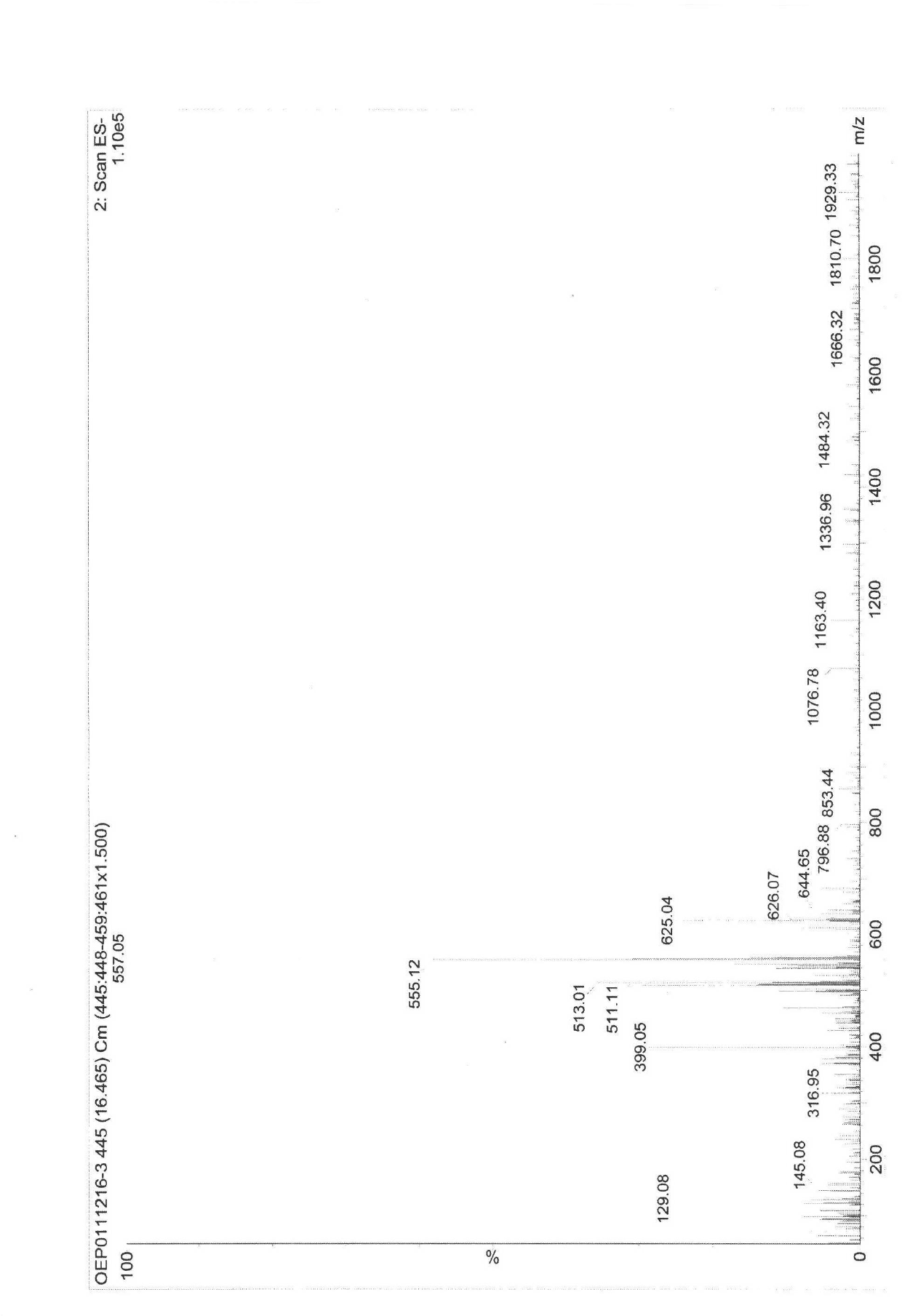
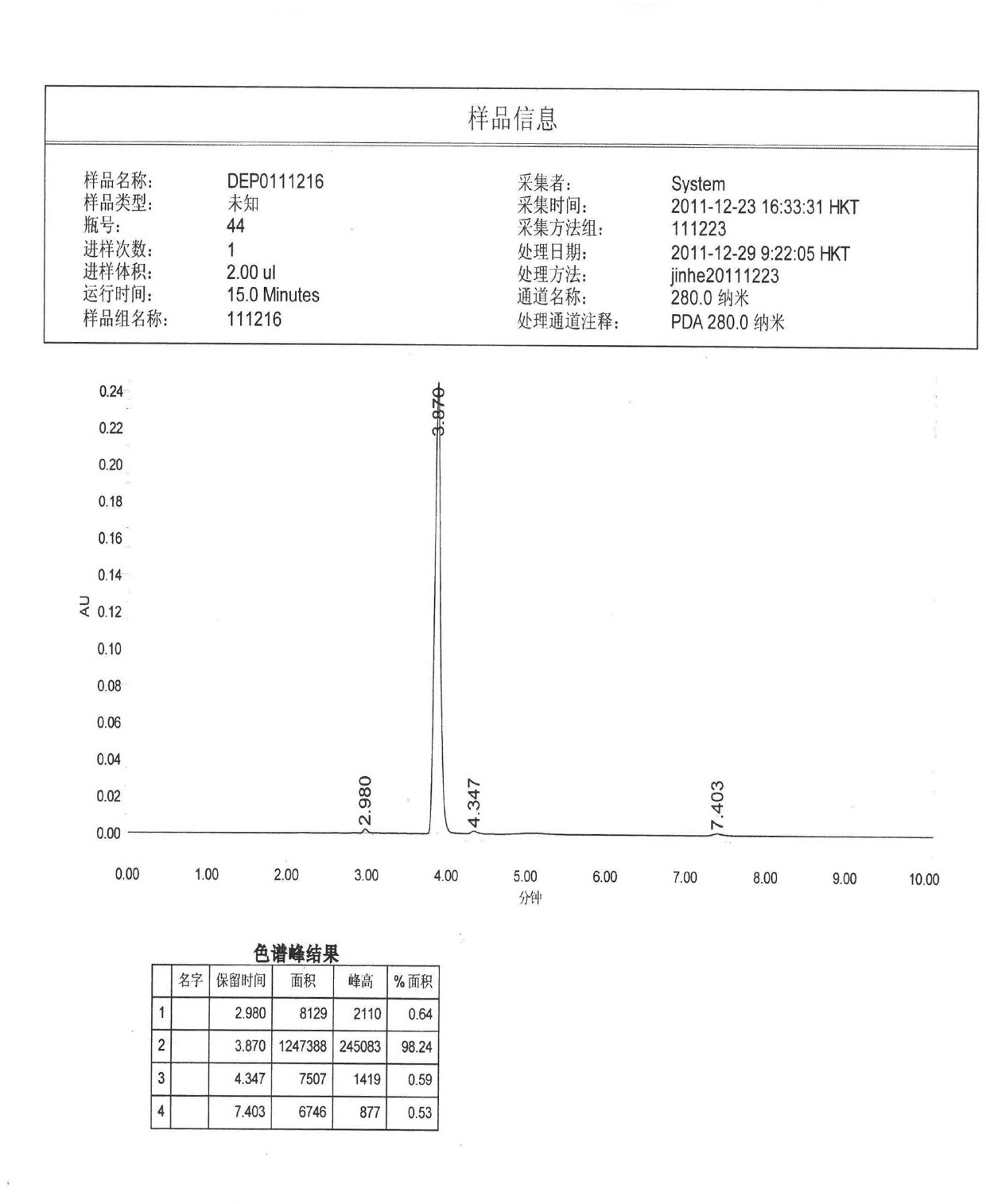
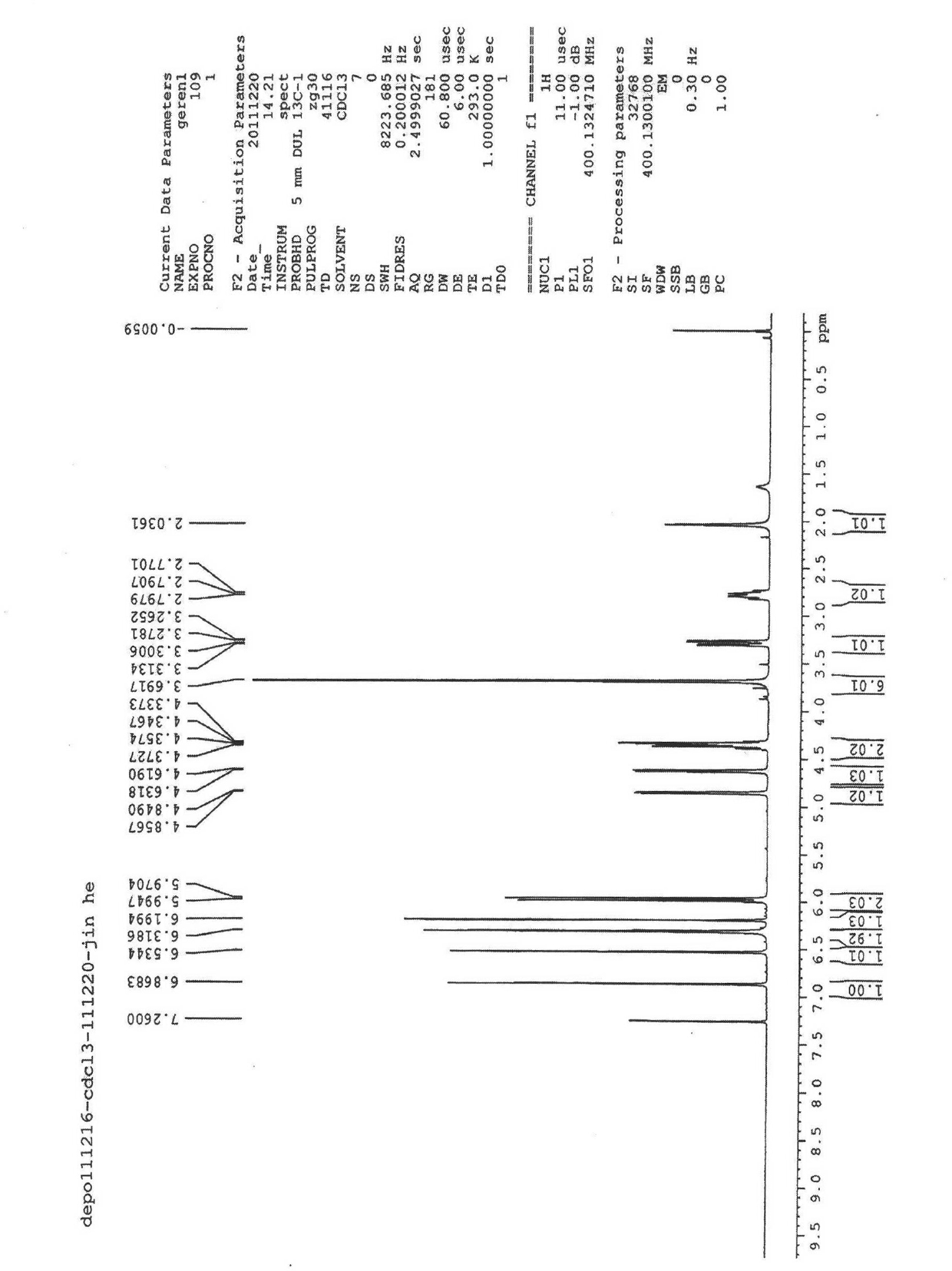
Image
Examples
Embodiment 1
[0042] (1) Preparation of compound B:
[0043] Drop into compound A (podophyllotoxin, the same below) (10kg) and dry dichloromethane (45L) respectively in the clean and dry 200L reactor, stir, then feed nitrogen into the reactor for 20 minutes to replace the Air. The temperature of the reaction solution was lowered to -10°C, and a mixed solution of iodotrimethylsilane (14Kg) and dichloromethane (15L) was added dropwise, the time was controlled at 2 hours, and the temperature of the dropping process was controlled at -10°C. After the dropwise addition, the temperature was controlled at -10°C, and the reaction was incubated for 4 hours. Thin-layer chromatography (TLC) detected that the reaction raw materials were completely consumed. Take 20L petroleum ether and add it. A large amount of powdery solids precipitated. After stirring for 20 minutes, filter to obtain the compound B (4'-desmethyl-4-iodopodophyllotoxin, the same below) crude product 10.84kg, yield: 88%, take the filt...
Embodiment 2
[0057] (1) Preparation of Compound B
[0058]Add compound A podophyllotoxin (20kg) and dry dichloromethane (90L) respectively into a clean and dry 500L reactor, stir, then feed nitrogen into the reactor for 20 minutes to replace the air in the reactor. The temperature of the reaction solution was lowered to -10°C, and a mixed solution of iodotrimethylsilane (28Kg) and dichloromethane (30L) was added dropwise, the time was controlled at 2 hours, and the temperature of the dropping process was controlled at -5°C. After the dropwise addition, the temperature was controlled at -5°C, and the reaction was kept for 4 hours. Thin-layer chromatography (TLC) detected that the reaction raw materials were completely consumed. Take 40L petroleum ether and add it. A large amount of powdery solid precipitated out. After stirring for 20 minutes, filter to obtain the compound B crude product 21.92kg, yield: 89%, take the filtrate solid and directly react in the next step.
[0059] (2) Acylati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com