Preparation method of 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid
A technology of difluoromethoxybenzoic acid and cyclopropylmethoxy, which is applied in the field of preparation of the chronic obstructive pulmonary disease drug roflumilast, can solve problems such as difficult industrial scale-up production, and achieve great positive progress , easy to operate, great effect of practical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
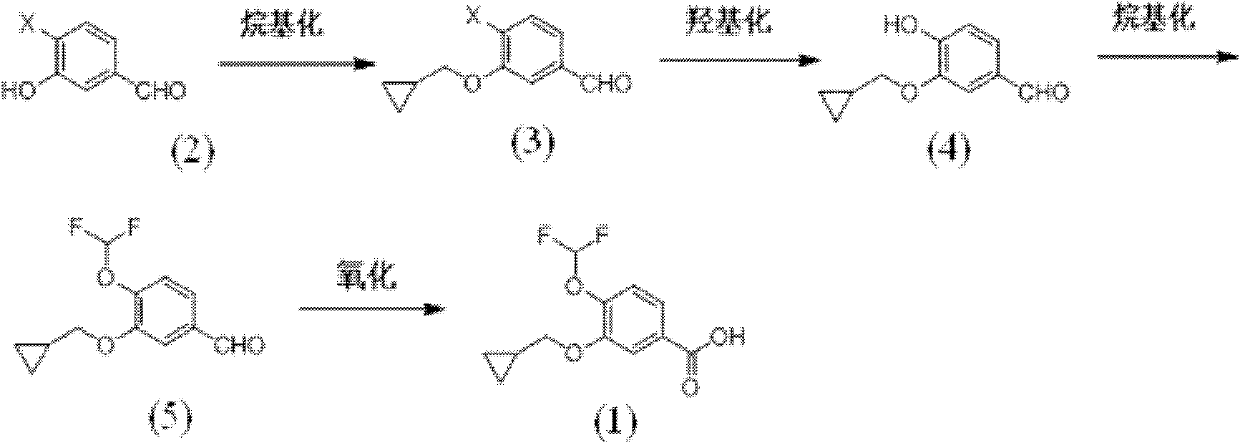
[0064] 3-Cyclopropylmethoxy-4-iodobenzaldehyde
[0065] 24.8 g of 3-hydroxy-4-iodobenzaldehyde, 27.6 g of potassium carbonate, 20.3 g of bromomethylcyclopropane, and 150 mL of DMF were stirred at 70° C. for 3 hours. After the reaction was diluted with 500 g of water, it was extracted with ethyl acetate, washed with water, and the organic layer was separated, washed with water, and dried. The solvent was distilled off under reduced pressure to obtain 30.1 g of 3-cyclopropylmethoxy-4-iodobenzaldehyde with a yield of 99%.
[0066] 1 H NMR (CDCl 3 ): δ0.40-0.47(m, 2H), 0.63-0.71(m, 2H), 1.23-1.40(m, 1H), 3.97(d, 2H), 7.38(d, 1H), 7.42(s, 1H ), 7.87 (d, 1H), 9.94 (s, 1H).
Embodiment 2
[0068] 3-Cyclopropylmethoxy-4-hydroxybenzaldehyde (4)
[0069] 1.9 g of cuprous iodide, 2.9 g of 8-hydroxypyridine, 100 mL of DMSO, and 30.1 g of methyl 3-cyclopropylmethoxy-4-iodobenzoate were added to the reactor. Under further stirring, 100 mL of a 25% potassium hydroxide aqueous solution was added.
[0070] Heated to 100°C for 24 hours, cooled to room temperature and filtered to remove the copper catalyst. Adjust to acidity with hydrochloric acid, extract with ethyl acetate, dry over anhydrous sodium sulfate, and evaporate the solvent under reduced pressure to obtain 17.8 g of 3-cyclopropylmethoxy-4-hydroxybenzaldehyde with a yield of 93%.
[0071] 1 H NMR (CDCl 3 ): δ0.37-0.41(m, 2H), 0.65-0.77(m, 2H), 1.25-1.40(m, 1H), 3.98(d, 2H), 7.00(d, 1H), 7.63(s, 1H ), 7.77(d, 1H), 10.2(s, 1H).
Embodiment 3
[0073] 3-Cyclopropylmethoxy-4-difluoromethoxybenzaldehyde (5)
[0074] Add 26.0g of potassium carbonate to a 100mL reactor containing DMF, raise the temperature to 80°C, and add 17.9g of 3-cyclopropylmethoxy-4-hydroxybenzaldehyde and 1-chloro-1,1-difluoro A solution of 28.4 g of sodium acetate in 50 mL of DMF was added and reacted for 2 hours. Cool down to room temperature, add 300 g of water to dilute, extract with ethyl acetate, dry over anhydrous sodium sulfate, and evaporate the solvent under reduced pressure to obtain 22.1 g of 3-cyclopropylmethoxy-4-difluoromethoxybenzaldehyde , yield 98%.
[0075] 1 H NMR (CDCl 3 ): δ0.38-0.41(m, 2H), 0.64-0.69(m, 2H), 1.28-1.32(m, 1H), 3.95(d, 2H), 6.72(t, 1H), 7.22(d, 1H ), 7.55 (s, 1H), 7.68 (d, 1H), 10.1 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com