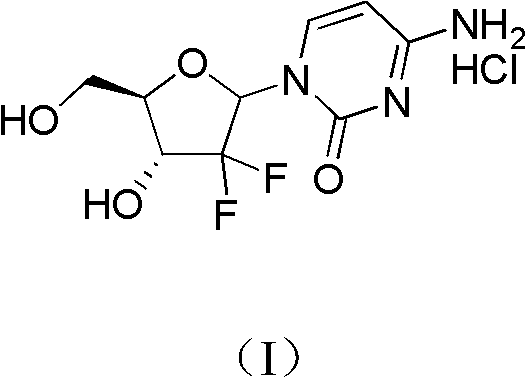
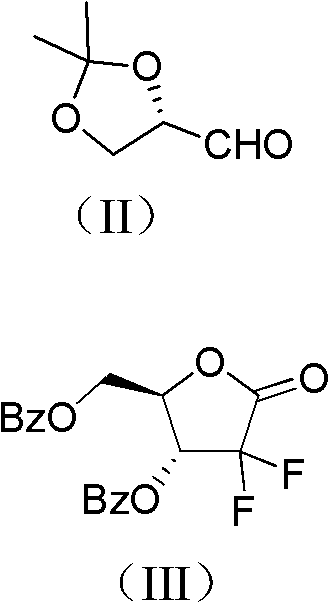
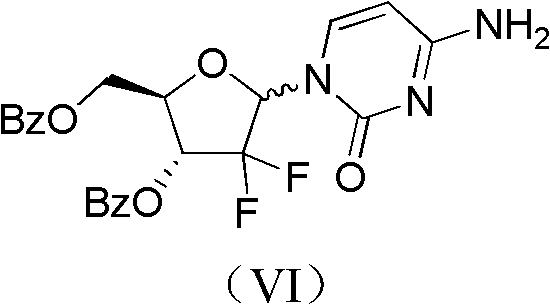
Method for preparing gemcitabine hydrochloride
A technology of concentrated hydrochloric acid and salt formation, which is applied in the field of preparation of anti-tumor active compounds, can solve the problems of harsh reaction conditions, low selectivity, and unsuitability for industrial production, and achieve mild reaction conditions, high reaction efficiency, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] In order to describe the contents and advantages of the present invention more specifically, the present invention will be further described below in conjunction with specific examples, but the scope of the present invention is not limited to the specific embodiments.
[0030] 1 Preparation of Intermediate A
[0031] Under the protection of nitrogen, the activated Zn powder (261.5g, 4.00mol), trimethylchlorosilane (51mL, 0.4mol), THF (600mL, dried on 4A molecular sieve) were mixed in a 3L three-necked flask, and stirred at room temperature. Weigh ethyl difluorobromoacetate (369.1g, 1.82mol), S-glyceraldehyde acetonide (260.0g, 2.00mol), THF (400ml, dried with 4A molecular sieve), mix thoroughly and drop a small amount of this solution to the In the three-necked bottle of Zn powder, after heating and triggering with a hair dryer, add the remaining solution dropwise, control the rate of addition to keep the system slightly boiling, and continue the heat preservation react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com