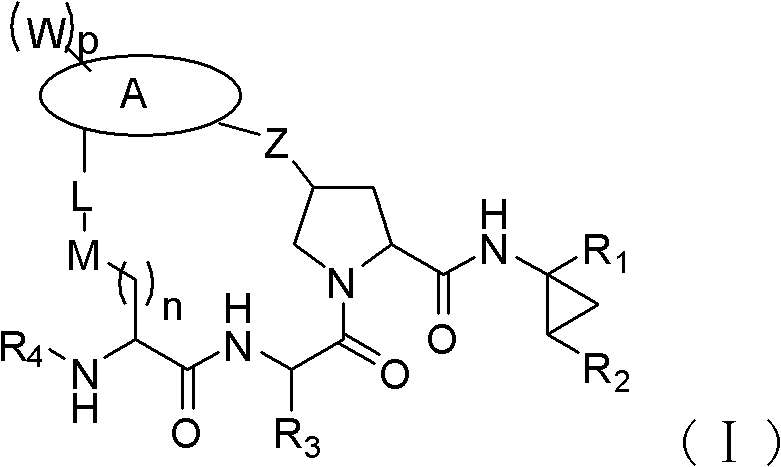
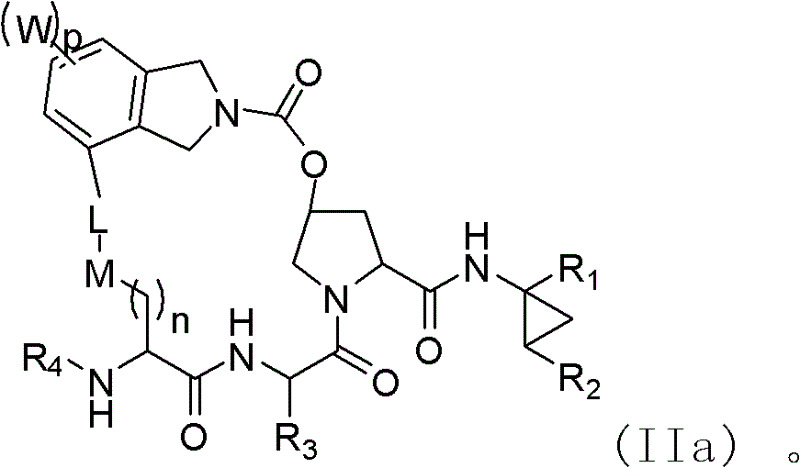
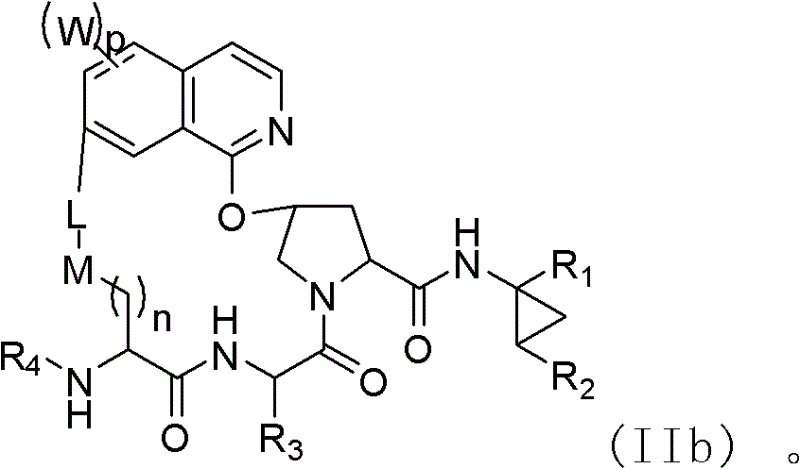
Macrocyclic compound for suppressing replication of hepatitis c viruses
A compound and solvate technology, applied in antiviral agents, enzyme inhibitor components, drug combinations, etc., can solve problems such as low living activity, large therapeutic dosage, and general pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Example 1 Compound IIa-1
[0152] N-[(1R, 12E, 17S, 20S, 23S)-20-tert-butyl-23-{[(1R, 2S)-1-[(cyclopropylsulfone)carbamoyl]-2-vinyl Cyclopropyl]carbamoyl}-3,18,21-trioxo-2,15-dioxa-4,19,22-triazatetracyclo[20.2.1.1 4,7 .0 6,11 ]Tert-Butyl Carbamate
[0153]
[0154] According to scheme I, III, the method for IV is synthesized.
[0155] Intermediate A3: (2S,4R)-4-(4-Vinylisoindole-2-carbonyloxy)pyrrolidine-1-carboxylic acid tert-butyl 2-methyl carboxylate
[0156]
[0157] According to Scheme I, starting material A2 (2.21 g, 9.03 mmol) was dissolved in DMF (20 mL), and CDI (1.47 g, 9.03 mmol) was added in portions at 0°C. After stirring at room temperature for 18 hours, slowly add the DMF solution of A1 (prepared with reference to the literature, J.Me d.Chem.2010, 53, 2443-2463) (1.4g, 9.03mmol), and stir after heating to 60°C after dropping 2 hours. After cooling to room temperature, ice water and 5% KHSO4 (35 mL) solution were added successively, and then e...
Embodiment 2
[0206] Example 2 Compound IIa-2
[0207] (1R, 12E, 17S, 20S, 23S)-17-amino-20-tert-butyl-N-[(1R, 2S)-1-[(cyclopropylsulfone)carbamoyl]-2-vinyl Cyclopropyl]-3,18,21-trioxo-2,15-dioxa-4,19,22-triazatetracyclo[20.2.1.1 4,7 .0 6,11 ] Hexadecyl-6,8,10,12-tetraene-23-carboxamide
[0208]
[0209] Compound IIa-1 (10mg, 0.012mmol) was dissolved in dichloromethane (5mL), then added dropwise into trifluoroacetic acid (1mL, 20%), stirred at room temperature for 2 hours, the reaction was completed, after concentration, water and di Chloromethane, and adjust the pH to 12 with 2N NaOH, and extract three times. The organic phase was washed with water, washed with saturated brine, Na 2 SO 4 Drying, filtration and concentration, white solid IIa-2 (2.3 mg, 26.1%) after column chromatography.
[0210] ESI-MS m / z 727.00(M+H) + .
Embodiment 3
[0211] Example 3 Compound IIa-3
[0212] N-[(1R, 12E, 17R, 20S, 23S)-20-tert-butyl-23-{[(1R, 2S)-1-[(cyclopropylsulfone)carbamoyl]-2-vinyl Cyclopropyl]carbamoyl}-3,18,21-trioxo-2,15-dioxa-4,19,22-triazatetracyclo[20.2.1.1 4,7 .0 6,11 ]Tert-Butyl Carbamate
[0213]
[0214] According to the method in Example 1, compound IIa-3 can be prepared from N-Boc-D-serine methyl ester.
[0215] 1 H NMR (400MHz, CDCl 3 )δ9.97 (brs, 1H), 7.28-7.08 (m, 3H), 6.55 (d, J=17.6Hz, 1H), 5.90-5.65 (m, 3H), 5.52 (s, 1H), 5.28-5.14 (m, 2H), 4.82-4.60(m, 6H), 4.47-4.26(m, 6H), 3.94-3.79(m, 2H), 3.42-3.32(m, 1H), 2.92-2.82(m, 1H) , 2.50-2.36(m, 2H), 2.11-1.96(m, 3H), 1.52(s, 9H), 1.35-1.30(m, 3H), 1.06(s, 9H), 1.06-1.02(m, 2H) ,; ESI-MS m / z 849.00 (M+Na) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com