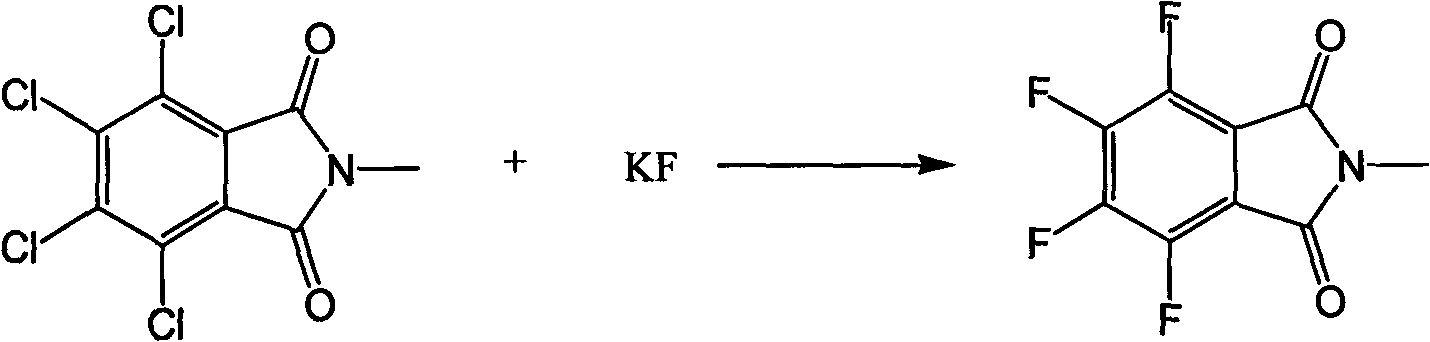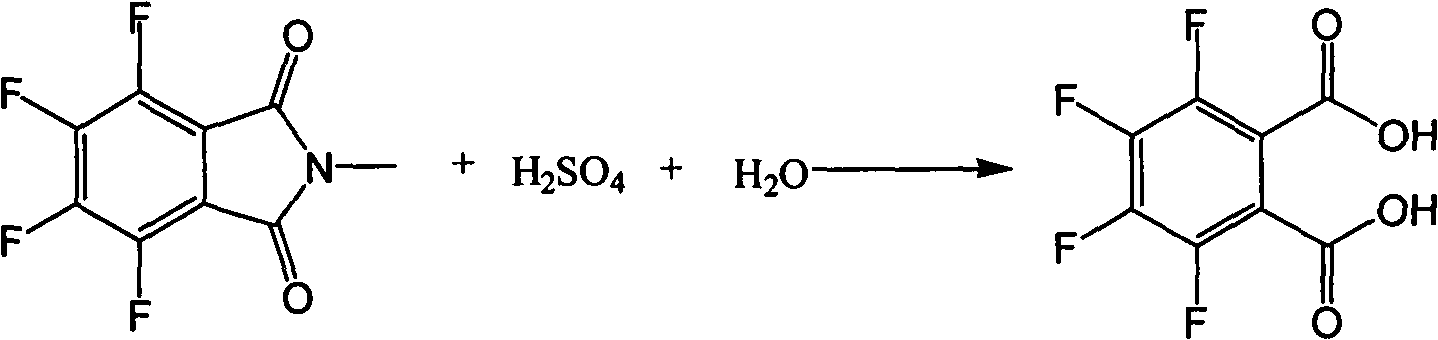Preparation method of 2,3,4,5-tetrafluorobenzoyl chloride
A technology of tetrafluorobenzoyl chloride and tetrafluorophthalic anhydride, which is applied in 2 fields, can solve the problems of high cost, complex process, low yield, etc., and achieve the goal of reducing raw material cost, reducing operation amount and improving yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
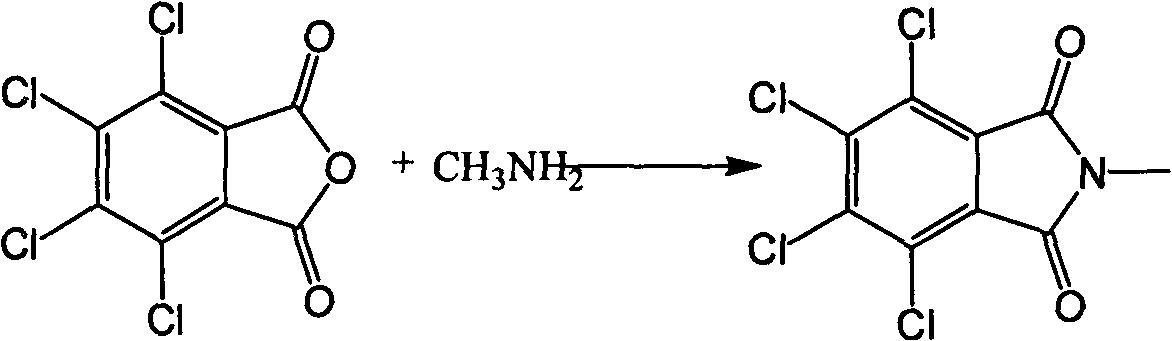
[0041] A preparation method of 2,3,4,5-tetrafluorobenzoyl chloride, comprising:
[0042] (1) Synthesis of N-methyl-3,4,5,6-tetrafluorophthalimide
[0043] In a 1000ml glass flask equipped with a thermometer, a water separator, a reflux condenser, and a mechanical stirrer, add 125g (0.437mol) of 3,4,5,6-tetrachlorophthalic anhydride, 200g of toluene, and 500g of sulfolane in sequence , 37.3 g (0.481 mol) of 40% aqueous methylamine solution was heated to reflux, and the moisture in the reaction was separated. After the moisture is exhausted, the reflux is maintained for 2 hours. The toluene was distilled off under reduced pressure, and the inner temperature was controlled below 80°C until almost no distillate was distilled out. Then 145 g (2.5 mol) of potassium fluoride was added, and the temperature was raised to 180° C. to react for 10 hours. After the reaction was completed, the sulfolane and the product were distilled off under reduced pressure, and then the distilled liq...
Embodiment 2
[0050] (1) Change the sulfolane in step (1) of Example 1 to dimethyl sulfoxide, and the rest are the same as in step (1). After the treatment, 92.2 g (0.395 mol) of white solid was obtained, yield: 90.5%. Melting point: 158~161℃.
[0051] (2) In a 1000ml glass flask equipped with a thermometer, a water separator, a reflux condenser, and a mechanical stirrer, add 250g of water, 80g (0.34mol) of the product of the above step (1), and slowly add a concentration of 50% 250g of sulfuric acid aqueous solution, allow it to heat up naturally. After the addition, the temperature was raised to 110° C., and the reaction was carried out for 15 hours. The conversion rate was 100% as detected by the liquid phase. After the reaction, cool down to 10-15°C, and some materials will precipitate out, filter and rinse with cold water. An off-white solid was obtained.
[0052] (3) Synthesis of 2,3,4,5-tetrafluorobenzoic acid
[0053] In a 1000ml glass flask equipped with a thermometer, a water ...
Embodiment 3
[0056] (1) Change the sulfolane in embodiment 1 step (1) into N, N-dimethylformamide, when 3,4,5,6-tetrachlorophthalic anhydride is 125g (0.437mol), The molar ratio of 3,4,5,6-tetrachlorophthalic anhydride to methylamine aqueous solution was adjusted to 1:1.5, and the rest was the same as step (1). After treatment, a white solid was obtained.
[0057] (2) in the 1000ml glass flask that thermometer, water separator, reflux condenser, mechanical stirrer are housed, add 100g water, product 80g (0.34mol) of above-mentioned steps (1), slowly add 250g of the vitriol oil, Let it warm up naturally. After the addition, the temperature was raised to 150° C., and the reaction was carried out for 5 hours, and the conversion rate was 100% as detected by the liquid phase. After the reaction, cool down to 10-15°C, and some materials will precipitate out, filter and rinse with cold water. An off-white solid was obtained.
[0058] (3) Synthesis of 2,3,4,5-tetrafluorobenzoic acid
[0059] I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com