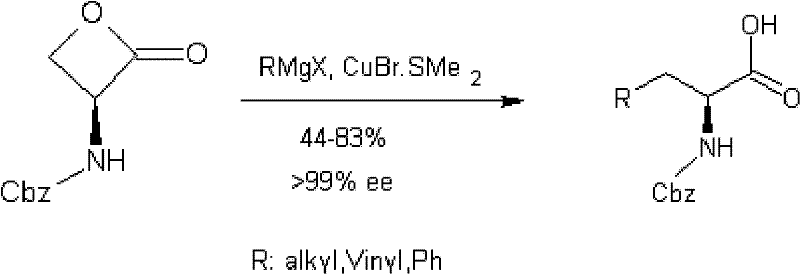Method for synthesizing carbobenzoxyserine-beta-lactone
A technology for synthesizing benzyloxycarbonylserine and benzyloxycarbonylserine, which is applied in the field of synthesis of chiral unnatural amino acids, can solve the problems of a large number of synthesis limitations, difficult product separation, difficult scale-up production, etc., and achieve low process reaction temperature requirements, The effect of high product purity and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A synthetic method for benzyloxycarbonylserine-β-lactone, which uses benzyloxycarbonyl-3-amino-alanine as raw material, reacts with sodium nitrite and citric acid aqueous solution to obtain benzyloxycarbonylserine-β-lactone ester. The reaction formula is as follows:
[0019]
[0020] The synthetic method of benzyloxycarbonyl-L-serine-β-lactone comprises the following steps:
[0021] Dissolve 11.9 grams of benzyloxycarbonyl-L-3-aminoalanine and 40 grams of citric acid in 300 milliliters of water, then cool to 0-5 ° C, add dropwise 60 milliliters of aqueous solution containing 3.45 grams of NaNO2, and keep the temperature at 0-5 5°C, add dropwise for about half an hour, then continue to react at 5°C for 2 hours, add ethyl acetate to extract, wash with 5wt% sodium bicarbonate, wash with water, dry, concentrate and crystallize to obtain 4.1 g of the product.
[0022] Melting point: 133-134°C [α] 20D: -25.5° (C=1, acetonitrile)
[0023] 'H NMR (300MHz, CDC13) 7.50-7.10...
Embodiment 2
[0025] A method for synthesizing benzyloxycarbonyl-L-serine-β-lactone, the method comprising the following steps:
[0026] Dissolve 11.9 grams of benzyloxycarbonyl-L-3-aminoalanine and 50 grams of citric acid in 300 milliliters of water, then cool to 0-5 ° C, add dropwise 120 milliliters of aqueous solution containing 17.25 grams of NaNO2, and keep the temperature at 0- Add dropwise at 5°C for about 2 hours, then continue to react at 15-25°C for 10 hours, add ethyl acetate for extraction, wash with 5wt% sodium bicarbonate, wash with water, dry, concentrate and crystallize to obtain 5.8 grams of the product.
[0027] Melting point: 133-135°C [α] 20D: -25.0° (C=1, acetonitrile)
[0028] 'H NMR (300MHz, CDC13) 7.50-7.10 (m, 5H), 5.40-5.12 (m, 2H), 5.00-4.82 (m, 1H), 4.45-4.08 (m, 2H)
Embodiment 3
[0030] A method for synthesizing benzyloxycarbonyl-D-serine-β-lactone, the method comprising the following steps:
[0031] Dissolve 11.9 grams of benzyloxycarbonyl-D-3-aminoalanine and 40 grams of citric acid in 300 milliliters of water, then cool to 0-5 ° C, add dropwise 80 milliliters of aqueous solution containing 6.9 grams of NaNO2, and keep the temperature at 5- Add dropwise at 10°C for about 1 hour, then continue to react at 25°C for 6 hours, add ethyl acetate to dissolve the solid, wash with 5wt% sodium bicarbonate, wash with water, dry, concentrate and crystallize to obtain 4.7 g of the product.
[0032] Melting point: 132-134°C [α] 20D: +25.3° (C=1, acetonitrile)
[0033] 'H NMR (300MHz, CDC13) 7.50-7.10 (m, 5H), 5.40-5.12 (m, 2H), 5.00-4.82 (m, 1H), 4.45-4.08 (m, 2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com