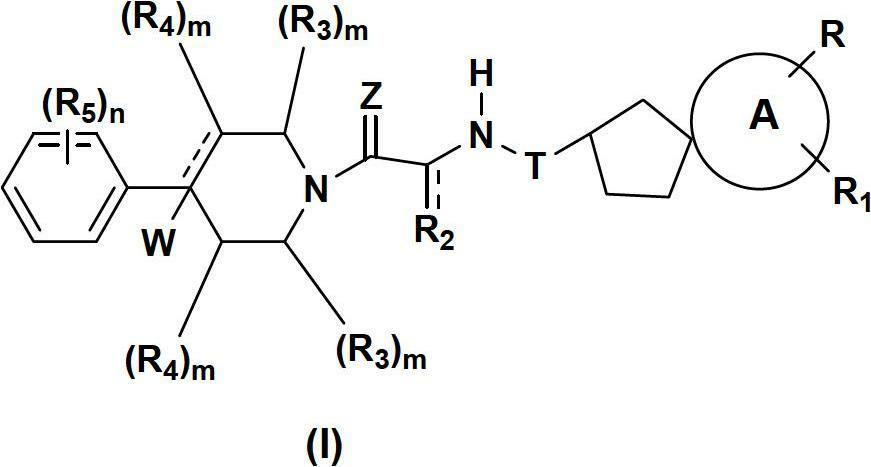
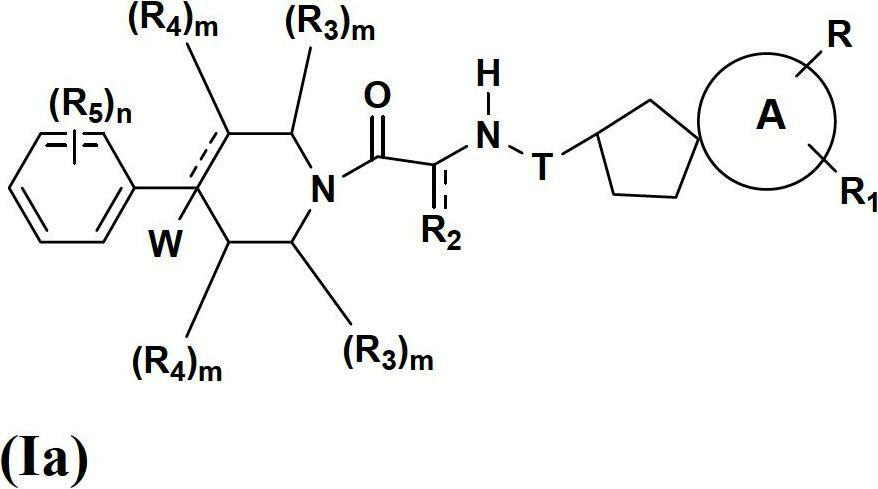
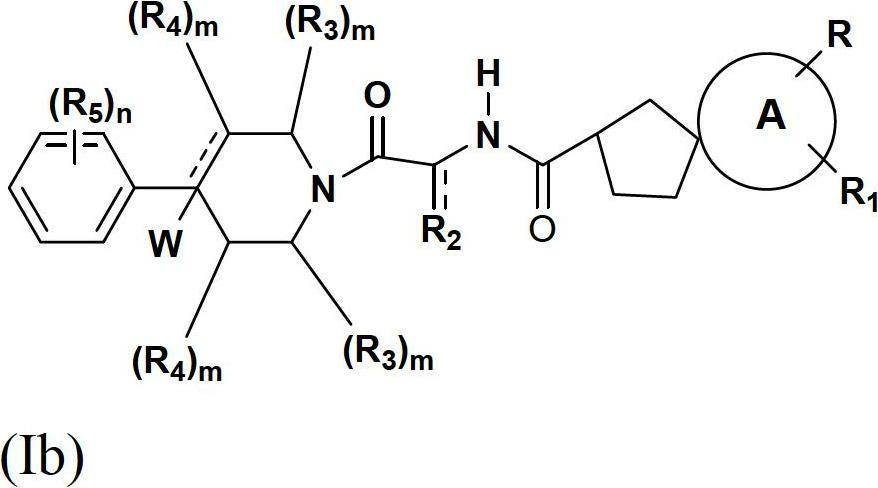
Spirocyclic compounds as modulators of chemokine receptor activity
A compound, cycloalkyl technology, applied in the fields of rheumatoid arthritis and transplant rejection, allergic diseases and autoimmune diseases, treatment and prevention of inflammatory diseases, can solve the problem of mobilizing monocytes and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] (2R)-N-((R)-1-((S)-4-(4-chlorophenyl)-4-hydroxy-3,3-dimethylpiperidin-1-yl)-3-methanol Base-1-oxo-but-2-yl)-7-oxo-8-oxa-6-azaspiro[4.5]decane-2-carboxamide
[0174]
[0175] Step A: (R)-N-((R)-1-((S)-4-(4-chlorophenyl)-4-hydroxy-3,3-dimethylpiperidin-1-yl)- 3-Methyl-1-oxo-but-2-yl)-3-oxocyclopentanecarboxamide
[0176]
[0177] To (R)-2-amino-1-((S)-4-(4-chlorophenyl)-4-hydroxyl-3,3-dimethylpiperidin-1-yl)-3-methanol at room temperature To a solution of butan-1-one hydrochloride (WO 2007092681, 300 mg, 0.799 mmol) in THF (2 mL) was added (R)-3-oxocyclopentanecarboxylic acid (WO 2007092681, 102 mg, 0.799 mmol) , triethylamine (0.223 mL, 1.599 mmol) and BOP (354 mg, 0.799 mmol). The resulting mixture was stirred at room temperature for 3 hours. The reaction mixture was concentrated and partitioned between ethyl acetate (15 mL) and 1N hydrochloric acid (1 mL). The organic layer was washed with water (3 mL), brine (2 mL), washed with anhydrous Na 2 SO 4 Drying,...
Embodiment 2
[0199] (2R)-N-((R)-1-((S)-4-(4-chlorophenyl)-4-hydroxy-3,3-dimethylpiperidin-1-yl)-3-methanol Base-1-oxo-but-2-yl)-7-oxo-6,8-diazaspiro[4.5]decane-2-carboxamide
[0200]
[0201] Step A: Benzyl (1R)-3-allyl-3-aminocyclopentanecarboxylate
[0202]
[0203] To a solution of benzyl (R)-3-oxocyclopentanecarboxylate (WO 2007092681, 1 g, 4.58 mmol) in methanol (5 mL) was added 2-allyl-4,4,5,5-tetra Methyl-1,3,2-dioxaborolane (1.155 g, 6.87 mmol) and ammonia solution (7N in MeOH, 8 mL). The resulting mixture was stirred at room temperature for 16 hours. The reaction mixture was concentrated and the residue was purified by preparative HPLC (Shimadzu VP-ODS 20x100 mm, 8 min gradient, solvent A: 10% MeOH, 90% H 2 O, 0.1% TFA; Solvent B: 90% MeOH, 10% H 2 O, 0.1% TFA; wavelength: 220 nM). The desired fractions were collected, concentrated and partitioned between ethyl acetate (20 mL) and saturated aqueous sodium bicarbonate (10 mL). The organic layer was washed with anhydrous...
Embodiment 3
[0223] N-((R)-1-((S)-4-(4-chlorophenyl)-4-hydroxy-3,3-dimethylpiperidin-1-yl)-3-methyl-1- Oxo-but-2-yl)-7-oxo-6-oxa-8-azaspiro[4.5]decane-1-carboxamide
[0224]
[0225] Step A: Benzyl 2-oxocyclopentanecarboxylate
[0226]
[0227]To ethyl 2-oxocyclopentanecarboxylate (4 g, 25.6 mmol) was added benzyl alcohol (3.6 g, 33.3 mmol) and the resulting mixture was heated to 175° C. for 1 hour. The reaction mixture was concentrated under reduced pressure using a high vacuum pump to obtain benzyl 2-oxocyclopentanecarboxylate (5.2 g, 23.84 mmol, 93% yield) as an oil. This compound was used in the next step without further purification.
[0228] Step B: Benzyl 2-allyl-2-hydroxycyclopentanecarboxylate (homochiral)
[0229]
[0230] 3-Bromoprop-1-ene (8.31 g, 68.7mmol) and indium (1.081ml, 68.7mmol). The resulting mixture was stirred at room temperature for 16 hours. The reaction mixture was concentrated and 1N hydrochloric acid (15 mL) was added to the residue and stirred a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com