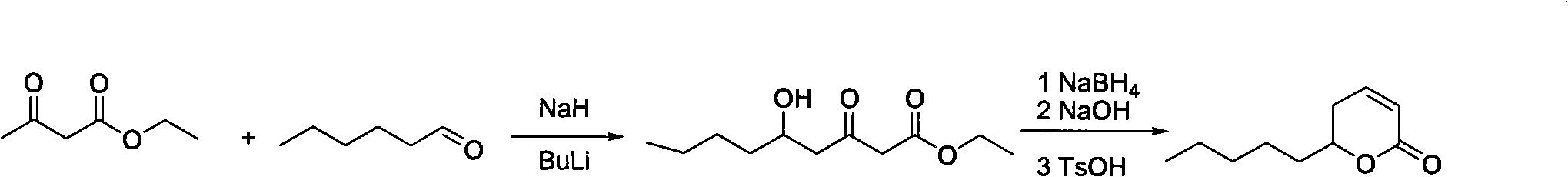
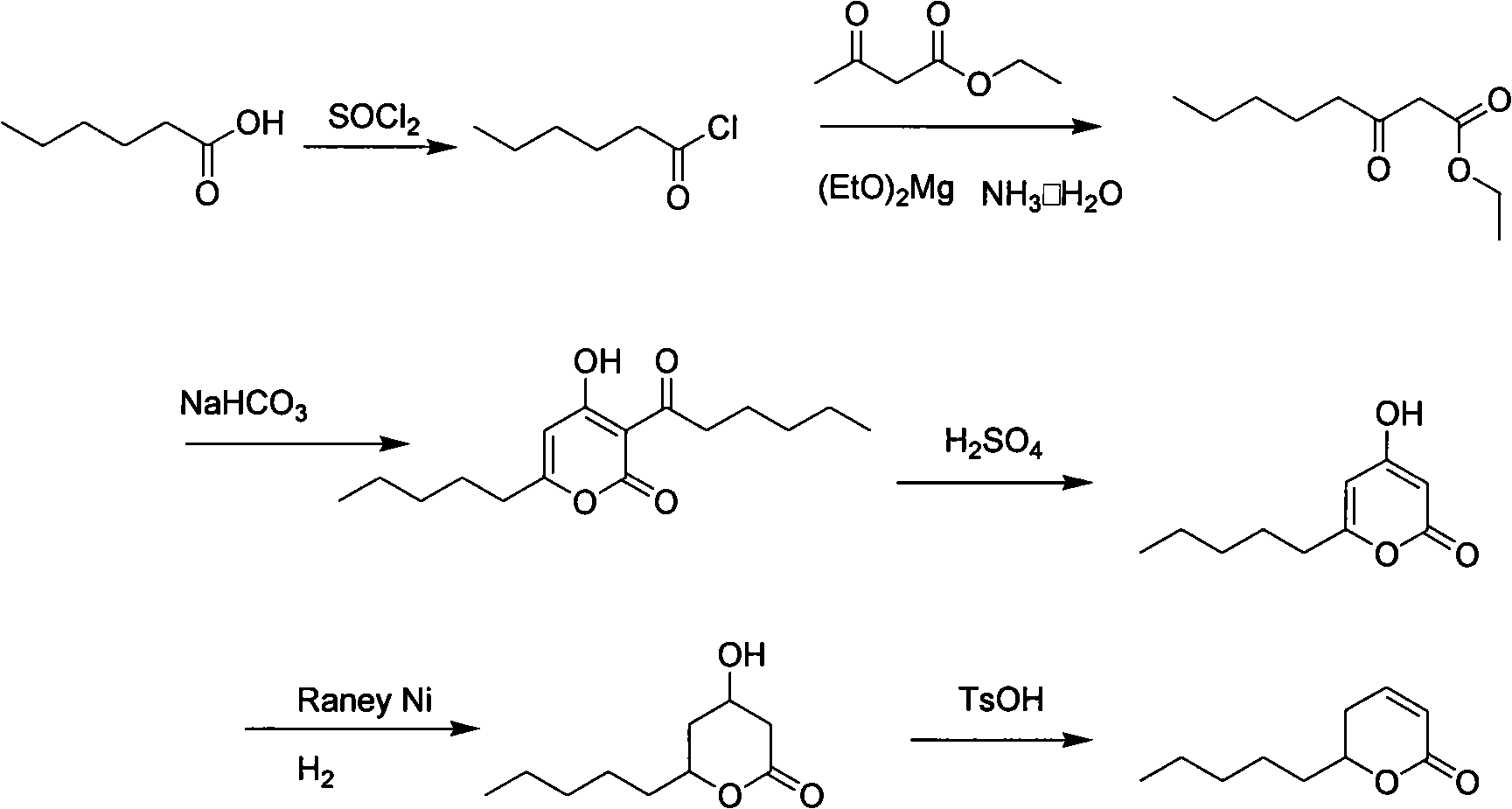
Synthesis method of massoia lactone
A technique of massoia lactone and a synthetic method, applied in the direction of organic chemistry and the like, can solve the problems of no commercial value, high cost of raw materials, cumbersome steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1: Preparation of Ethyl Hexanoacetate
[0030] In a 5000ml three-neck flask equipped with mechanical stirring, a thermometer and a reflux tube, add 300g of ethyl acetoacetate, 3L of toluene, and 360g of magnesium ethoxide in sequence, react at room temperature for 1 hour, heat to reflux, and slowly add 350g of hexanoyl chloride dropwise for about 2 hours After dripping, add 200ml of 5% HCl after cooling, wash with 50ml of saturated sodium chloride, add 800ml of 25% ammonia water, react at room temperature for 2 hours, separate liquids, wash with dilute hydrochloric acid for 1 hour, wash with saturated sodium chloride, and water. Recover toluene, distill under reduced pressure, collect the components at 87-92 degrees / 1mmHg to obtain ethyl hexanoyl acetate with a yield of 82%.
[0031] Step 2: Preparation of 3-hexanoyl-6-pentyl-2H-pyran-2,4(3H)dione
[0032] Into a 2000mL three-necked flask, add 1000g of ethyl hexanoacetate obtained in step (1), 0.1g of sodium bicar...
Embodiment 2
[0043] Step 1: Preparation of Ethyl Hexanoacetate
[0044]In a 5000ml three-necked flask equipped with mechanical stirring, a thermometer and a reflux tube, add 300g of ethyl acetoacetate, 3L of tetrahydrofuran, and 380g of magnesium ethoxide in sequence, react at room temperature for 1 hour, heat to reflux, and slowly add 350g of hexanoyl chloride dropwise for about 2 hours After dripping, add 200ml of 5% HCl after cooling, wash with 50ml of saturated sodium chloride, then add 1000ml of 25% ammonia water, react at room temperature for 2 hours, separate liquids, wash with dilute hydrochloric acid for 1 hour, wash with saturated sodium chloride, and water. Recover toluene, distill under reduced pressure, collect 87-92 degree / 1mmHg component, yield 80%.
[0045] Step 2: Preparation of 3-hexanoyl-6-pentyl-2H-pyran-2,4(3H)dione
[0046] Add 1000g of ethyl hexanoacetate and 0.05g of sodium bicarbonate to a 2000mL three-necked flask, replace with nitrogen, react in a nitrogen strea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com