Catalyst for producing aldehyde through hydroformylation of alkene and application thereof
A technology for alkene hydroformyl and catalyzing alkene hydroformyl, which is applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, carbon monoxide reaction preparation, etc., can solve the problem that the activity of rhodium phosphine catalyst needs to be improved, Problems such as poor catalyst stability, to achieve the effect of simple separation, good catalytic performance, and guaranteed stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
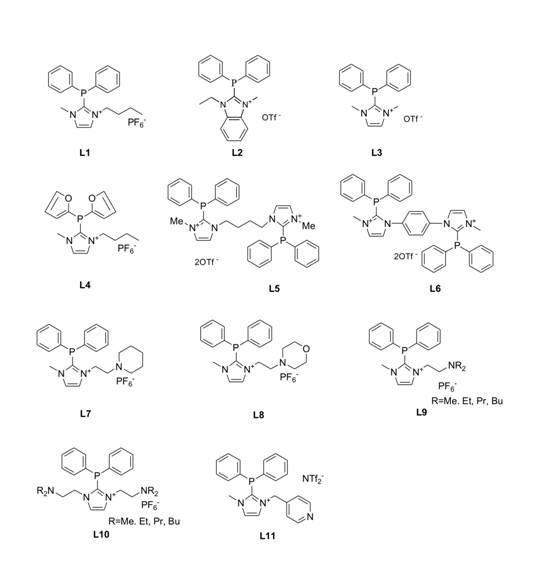
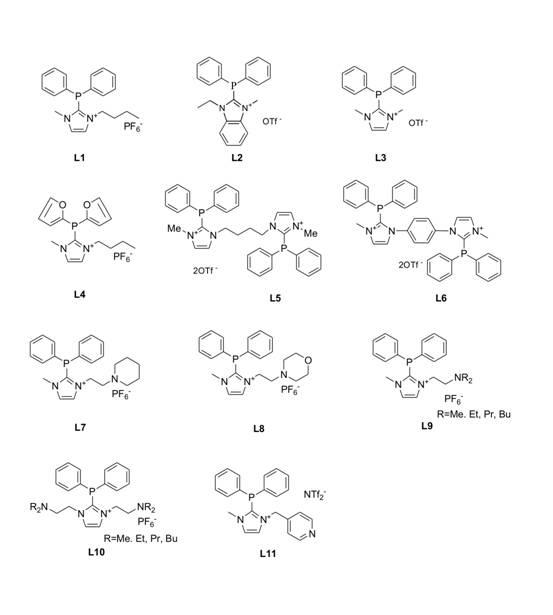
[0021] Example 1: L1 Synthesis:
[0022] 1-Butyl-3-methylimidazolium hexafluorophosphate was dissolved in dry tetrahydrofuran, under nitrogen protection, at -78°C, 1.05 equivalents of n-butyllithium ( n -BuLi), reacted for 1 hour after the drop, then slowly added 1.05 equivalents of diphenylphosphine chloride dropwise, and slowly rose to room temperature overnight after the drop. The reaction solution was washed with water, dried over anhydrous magnesium sulfate, crystallized with dichloromethane and absolute ethanol, and dried in vacuum to obtain white crystals which were ligands L1 , with a structure such as L1 shown.
Embodiment 2
[0023] Example 2: L2 Synthesis:
[0024] N-ethylbenzimidazole was dissolved in dry tetrahydrofuran, and 1.05 equivalents of TMEDA and n -BuLi, reacted for 1 hour, then added diphenylphosphine chloride dropwise, slowly raised to room temperature after dropping, stirred overnight, and separated by column chromatography to obtain a yellow viscous liquid. The yellow liquid was dissolved in dry dichloromethane. Under nitrogen protection and at -78°C, 1.05 equivalents of methyl trifluoromethanesulfonate was added dropwise. After the drop was completed, it was slowly raised to room temperature, stirred overnight, and the solvent was removed by rotary evaporation. Washed with ether to give a white solid L2 , with a structure such as L2 shown.
Embodiment 3
[0025] Example 3: L3 Synthesis:
[0026] L3 synthetic method and L2 The synthetic method of is the same, and the starting material is replaced by N-methylimidazole. structure like structure L3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com