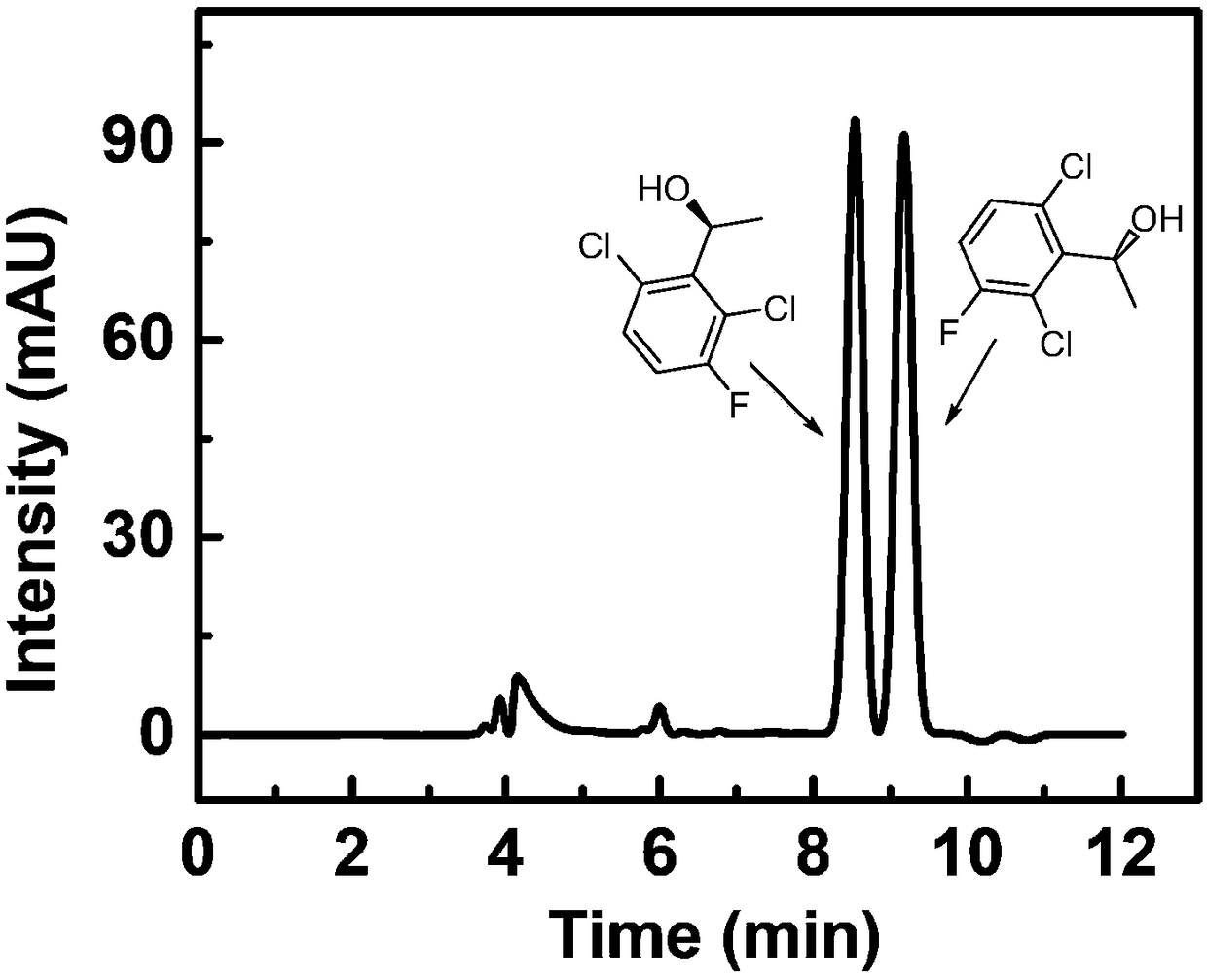
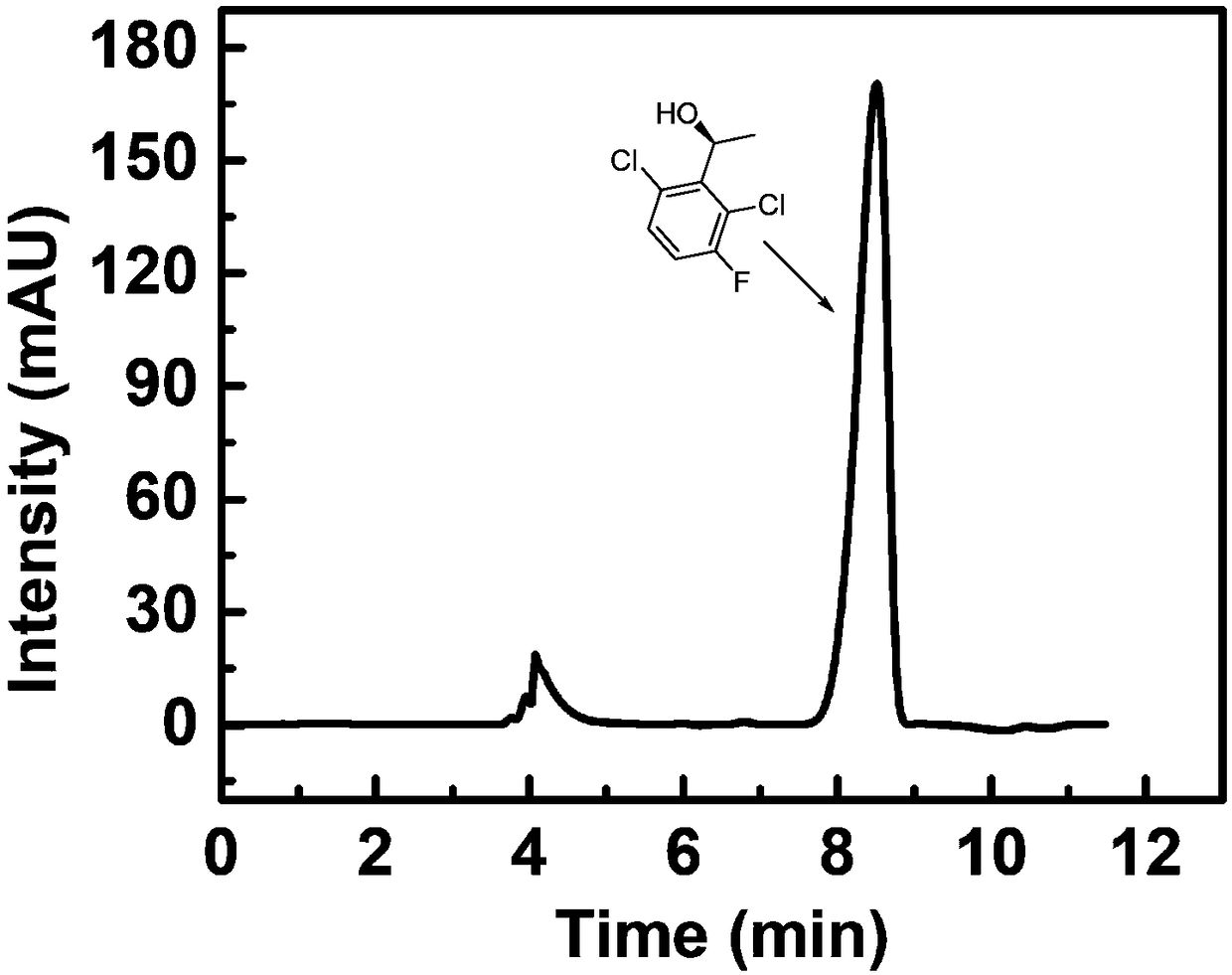
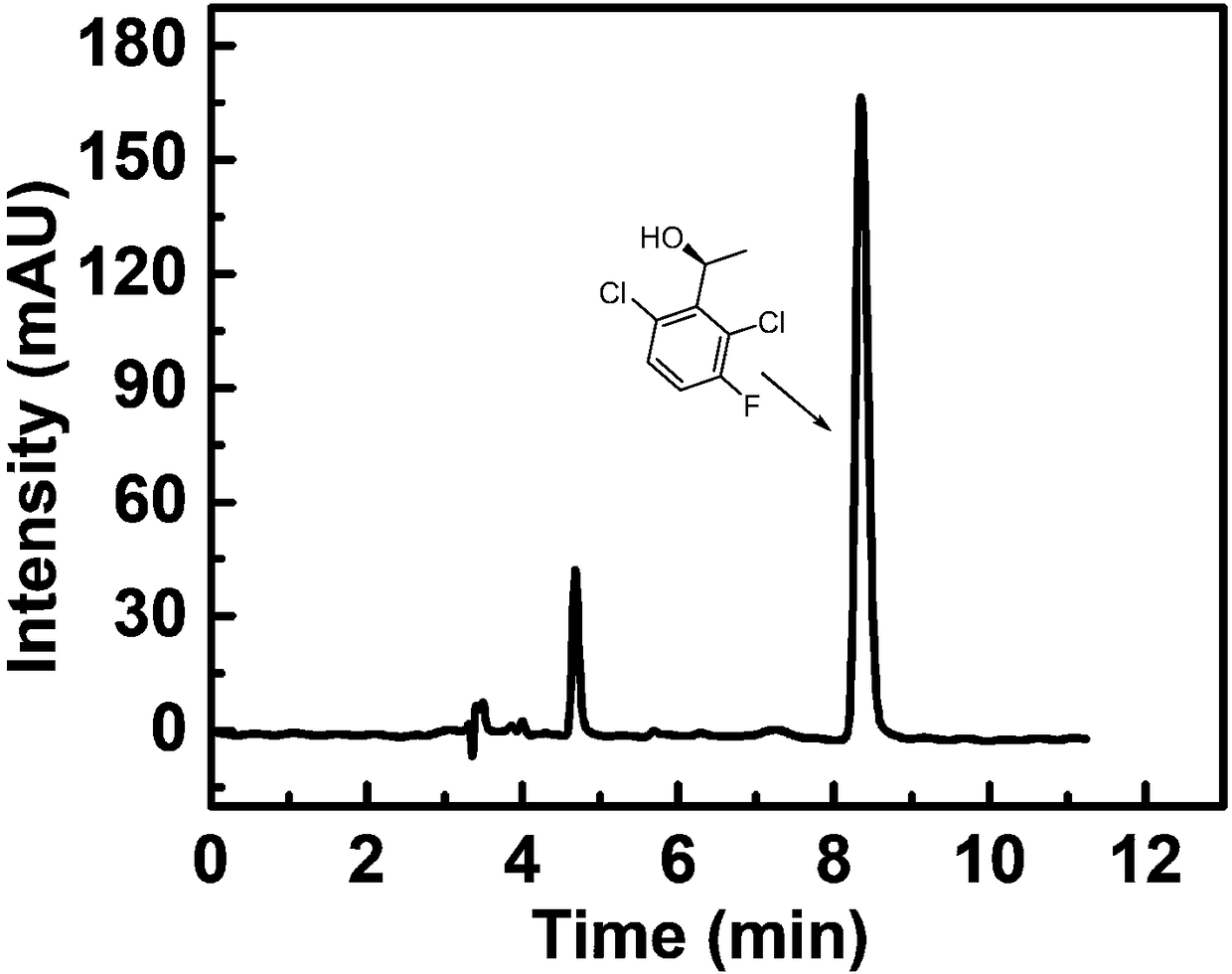
Method for synthesizing (S)-1-1(2,6-dichloro-3-fluorine-phenyl) ethanol through immobilized bienzyme catalysis
A fluoroacetophenone and double-enzyme technology, which is applied in the field of immobilized double-enzyme catalyzed synthesis of 1-ethanol, can solve the problems that the catalyst, that is, the free enzyme is unstable and inconvenient, the optical purity of the product needs to be improved, and the catalyst consumption is large, etc. The effect of low cost, high product yield and simple recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 4mg of 2,6-dichloro-3-fluoroacetophenone and 5mg of coenzyme NADP + , dissolved in the reaction solvent, the reaction solvent is 90% potassium phosphate buffer and 10% isopropanol. Then add calcium phosphate enzyme crystals to the reaction solvent, the enzyme crystals contain 3 mg of aldehyde ketone reductase and 1 mg of alcohol dehydrogenase, place the reaction container in a shaking table at 40 ° C for 12 hours, and filter after the reaction is over. The filter residue is washed with a washing solvent to obtain a filtrate, and then water and n-heptane are added to the filtrate for extraction to obtain an organic layer and an aqueous layer. The washing solvent is the same as the extraction solvent, and the organic layer is dried by adding anhydrous magnesium sulfate, and filtered , heated and spin-dried under reduced pressure to obtain a solid. The product was analyzed by chiral HPLC, and the conversion rate was 95%, and its optical purity was 100% ee.
[0043]...
Embodiment 2
[0046] Weigh 10mg of 2,6-dichloro-3-fluoroacetophenone and 10mg of coenzyme NADP + , dissolved in the reaction solvent, the reaction solvent is 90% potassium phosphate buffer and 10% isopropanol. Then add calcium phosphate enzyme crystals to the reaction solvent, the enzyme crystals contain 10 mg of aldehyde ketone reductase and 3 mg of alcohol dehydrogenase, and place the reaction container in a shaker at 30°C for 12 hours of shaking; after the reaction, filter, The filter residue is washed with a washing solvent to obtain a filtrate, and then water and n-heptane are added to the filtrate for extraction to obtain an organic layer and an aqueous layer. The washing solvent is the same as the extraction solvent, and the organic layer is dried by adding anhydrous magnesium sulfate, and filtered , heated and spin-dried under reduced pressure to obtain a solid. The product was analyzed by chiral HPLC, and the conversion rate was 32%, and its optical purity was 100% ee.
Embodiment 3
[0048] Weigh 10mg of 2,6-dichloro-3-fluoroacetophenone and 10mg of coenzyme NADP + , dissolved in the reaction solvent, the reaction solvent is 90% potassium phosphate buffer and 10% isopropanol. Then add calcium phosphate enzyme crystals to the reaction solvent, the enzyme crystals contain 10 mg of aldehyde ketone reductase and 3 mg of alcohol dehydrogenase, and place the reaction container in a shaker at 40°C for 12 hours of shaking reaction; after the reaction, filter, The filter residue is washed with a washing solvent to obtain a filtrate, and then water and n-heptane are added to the filtrate for extraction to obtain an organic layer and an aqueous layer. The washing solvent is the same as the extraction solvent, and the organic layer is dried by adding anhydrous magnesium sulfate, and filtered , heated and spin-dried under reduced pressure to obtain a solid. The product was analyzed by chiral HPLC, and the conversion rate was 79%, and its optical purity was 100% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com