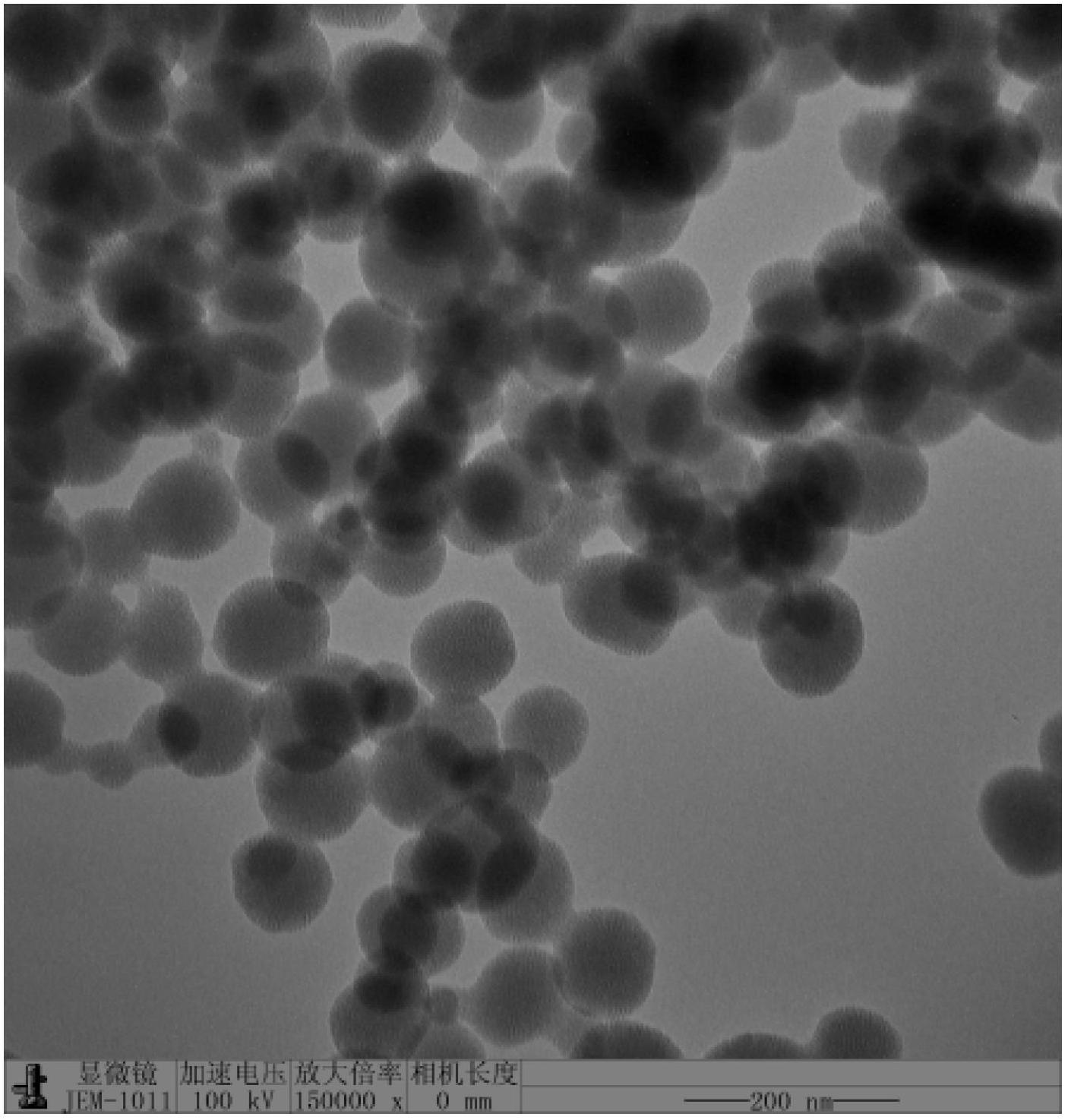
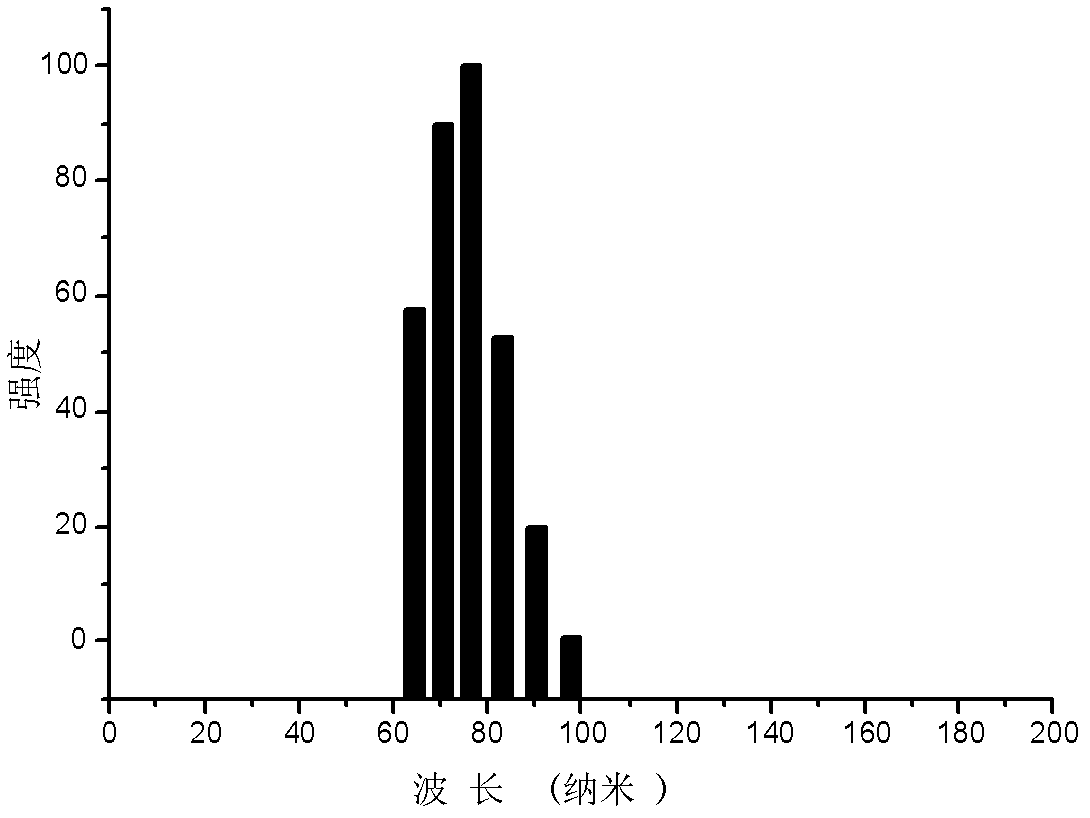
LHRH-bonded amphiphilic biodegradable polymer, preparation method and application
A biodegradable, LHRH-6-NH2 technology, applied in the field of biomedicine, can solve the problems of poor stability of LHRH, high cost, and difficulty in linking drug molecules with LHRH, and achieve reduction of toxic and side effects, low cost, high bioavailability and The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preparation method of the amphiphilic biodegradable polymer bound with LHRH is to prepare the polymer shown in formula a, when the LHRH polypeptide derivative is LHRH-10-COOH, the steps are as follows:
[0051] Dissolve LHRH-10-COOH in N,N-dimethylformamide or water to dissolve it, add 1-ethyl-(3-dimethylaminopropyl) carbonyl dicarboxylate with 1.5-3 times the molar number of carboxyl groups Imine hydrochloride (EDC·HCl) and 4-dimethylaminopyridine (DMAP) with 0.01 times the number of moles of carboxyl groups, stirred in ice bath for 10-30 minutes, and added with 1-3 times the number of moles of carboxyl groups containing terminal amino groups or terminal hydroxyl groups Two-block copolymer, kept in an ice bath and naturally raised to room temperature, stopped the reaction after 12-24 hours to obtain a reaction solution, placed the reaction solution in a dialysis bag with a molecular weight cut-off of 3500 g / mol and dialyzed with distilled water for 24-48 hours, The...
Embodiment 1
[0085] Example 1: Polypeptide targeting LHRH-6-NHCO-PEG 5K -PLA 2K Preparation of block copolymers
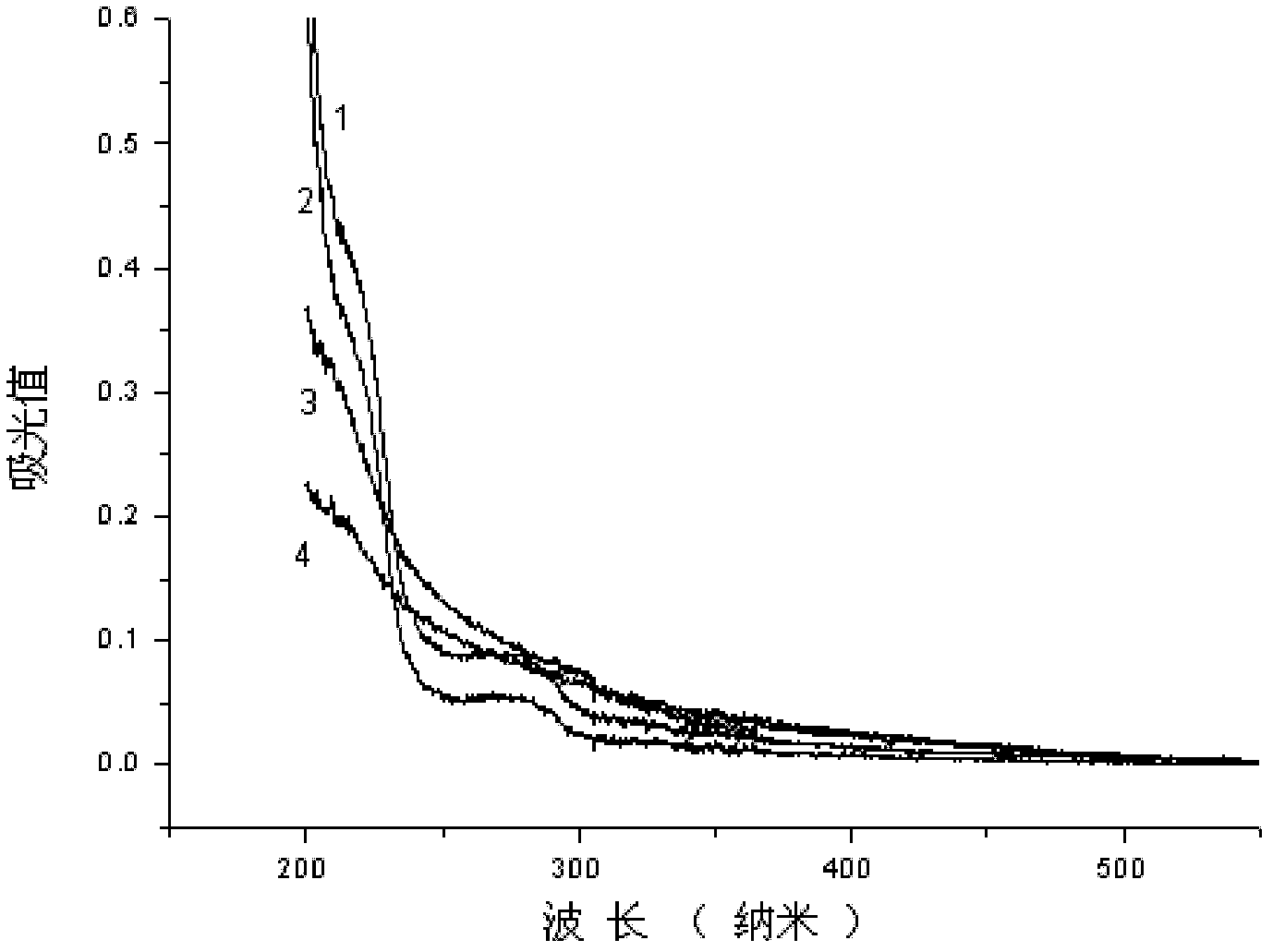
[0086] 0.5mmol carboxyl-terminated diblock copolymer COOH-PEG 5K -PLA 2K (The subscript number in the molecular formula represents the number-average molecular weight of the block, the same below), 0.61mg DMAP and 0.75mmol EDC·HCl are placed in a dry ampoule, add 10ml distilled water and stir for 30 minutes under ice-cooling, add 0.25mmolLHRH -6-NH 2 , kept in an ice bath and naturally raised to room temperature, stirred for 12 hours, placed in a dialysis bag with a molecular weight cut-off of 3500 g / mol, dialyzed with distilled water, changed the water every 6 hours, and lyophilized after 24 hours to obtain a white polypeptide targeting block copolymer LHRH-6-NHCO-PEG 5K -PLA 2K freeze-dried powder. figure 1 It is the ultraviolet-visible absorption spectrum of the block copolymer of bonded LHRH derivatives, and the curve 2 in the figure is the LHRH-6-NHCO-PEG of embodim...
Embodiment 2
[0087] Example 2: Polypeptide targeting LHRH-6-PEG 10K -PGA 20K Preparation of block copolymers
[0088] 1mmol carboxyl-terminated block polymer COOH-PEG 10K -PGA 20K , 1.22mg DMAP and 3mmol EDC·HCl were placed in a dry ampoule, and 10ml N,N-dimethylformamide was added under ice cooling to dissolve and stir for 30 minutes, and 1mmol LHRH-6-NH 2 , kept in an ice bath and naturally raised to room temperature, stirred for 24 hours, placed in a dialysis bag with a molecular weight cut-off of 3500g / mol, dialyzed with distilled water, changed the water every 12 hours, and freeze-dried after 48 hours to obtain a white polypeptide targeting block copolymer LHRH-6-PEG 10K -PGA 20K freeze-dried powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com