Preparation method for vanadium trioxide doped powder material
A technology of vanadium trioxide powder and powder materials, applied in the direction of vanadium oxide, etc., which can solve the problems of environmental pollution, high preparation cost, and unsuitability for large-scale industrial production, and achieve good product quality, simple raw materials, and large-scale production. The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
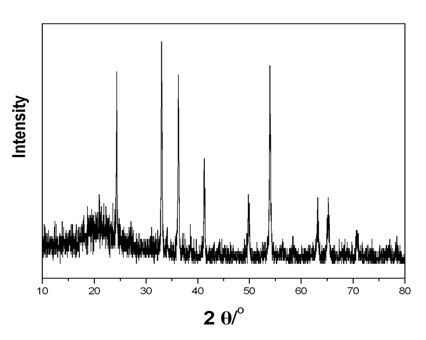
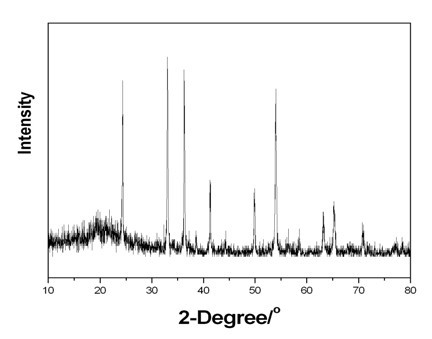
[0028] 0.234g ammonium metavanadate (NH 4 VO 3 ) and 0.126g oxalic acid (H 2 C 2 o 4 2H 2 0), was added to 30mL deionized water, and magnetically stirred until the solution became a uniform liquid. The obtained solution was transferred into a 50 mL hydrothermal kettle, and reacted for 48 hours at 180°C. After the reaction was completed, it was naturally cooled, and the obtained solid was centrifuged, washed and dried. Finally, the obtained solid powder was calcined at 700°C for 3 hours in an atmosphere of high-purity argon (99.999%) to obtain V 2 o 3 Powder material. The product was characterized by XRD as V 2 o 3 , see attached figure 1 , see attached table 1 for the phase transition temperature.
Embodiment 2
[0030] 0.234g ammonium metavanadate (NH 4 VO 3 ), 0.126g oxalic acid (H 2 C 2 o 4 2H 2 O) and 0.005g chromium chloride (CrCl 3 ·6H 2 O), added to 30mL of deionized water, and magnetically stirred until the solution became a homogeneous liquid. The obtained solution was transferred into a 50 mL hydrothermal kettle, and reacted for 48 hours at 180°C. After the reaction was completed, it was naturally cooled, and the obtained solid was centrifuged, washed and dried. Finally, the obtained solid powder was calcined at 700°C for 3 hours in an atmosphere of high-purity argon (99.999%) to obtain chromium-doped V 2 o 3 Powder material. The product was characterized by XRD as V 2 o 3 , see attached figure 1 , see attached table 1 for the phase transition temperature.
Embodiment 3
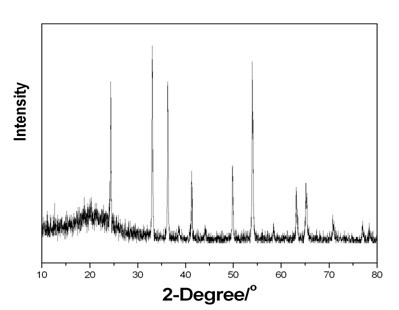
[0032] 0.234g ammonium metavanadate (NH 4 VO 3 ), 0.252g oxalic acid (H 2 C 2 o 4 2H 2 O) and 0.026g lanthanum nitrate (La(NO 3 ) 3 ·6H 2 O), added to 30mL of deionized water, and magnetically stirred until the solution became a homogeneous liquid. The obtained solution was transferred into a 50 mL hydrothermal kettle, and reacted for 168 h at 160° C. After the reaction was completed, it was naturally cooled, and the obtained solid was centrifuged, washed and dried. Finally, the obtained solid powder was calcined at 1000°C for 1 h in an atmosphere of high-purity argon (99.999%) to obtain lanthanum-doped V 2 o3 Powder material. The product was characterized by XRD as V 2 o 3 , see attached image 3 , see attached table 1 for the phase transition temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com