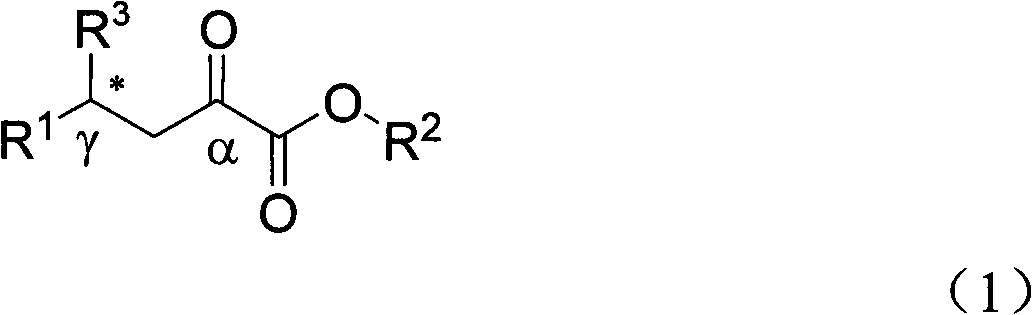
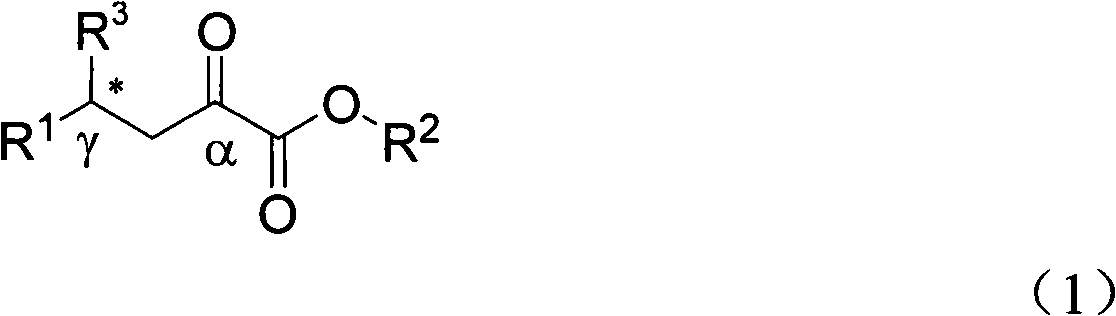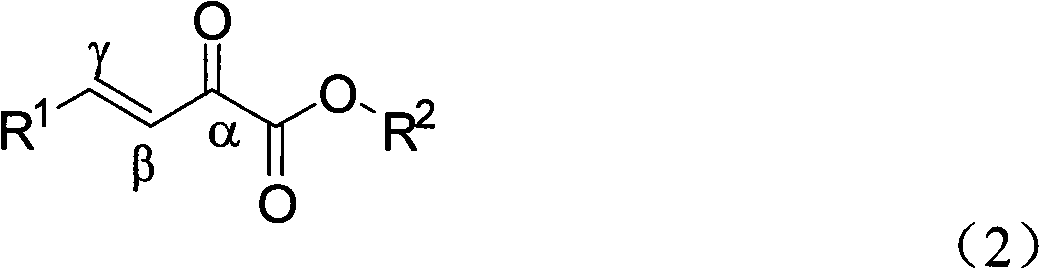Alpha-keto ester compound with gamma chiral center and synthetic method thereof
A synthetic method and technology of keto esters, applied in the direction of organic chemical methods, preparation of organic compounds, chemical instruments and methods, etc., to achieve good application prospects, good application effects, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The catalytic product 3a (R 2 = Me,R 1 =Ph) Preparation
[0033] 5mL reaction flask, add copper acetate monohydrate (0.005mmol) and ligand L3 (0.01mmol), N 2For protection, add 1 mL of dry toluene, stir and coordinate at room temperature for 3 hours, dissolve the substrate γ-phenyl β, γ-unsaturated α-keto acid methyl ester (0.25 mmol) in 1 mL of dry toluene and add dropwise to the reaction system and stir for 5 minute. Diethylzinc (0.375 mmol, 1.0 M in n-hexane) was slowly added dropwise. The system was reacted at 26°C, and the reaction was monitored by thin-layer chromatography (hereinafter referred to as "TLC"). After the reaction was completed for 2 hours, a saturated solution of ammonium chloride was added to quench, and ethyl acetate (5ml×3) extracted , dried over anhydrous magnesium sulfate, filtered and concentrated. Ethyl acetate / petroleum ether=1:10 The product 3a was obtained by column chromatography with a yield of 81% and corresponding selectivity of 58...
Embodiment 2
[0038] The catalytic product 3a (R 2 = Me,R 1 =Ph) Preparation
[0039] 5mL reaction flask, add copper acetate monohydrate (0.005mmol) and ligand L3 (0.01mmol), N 2 For protection, add 1 mL of dry toluene, stir and coordinate at room temperature for 3 hours, dissolve the substrate γ-phenyl β, γ-unsaturated α-keto acid methyl ester (0.25 mmol) in 1 mL of dry toluene and add dropwise to the reaction system and stir for 5 minute. Diethylzinc (0.375 mmol, 1.0 M in n-hexane) was slowly added dropwise. The system was reacted at -20°C, and the reaction was monitored by TLC. The reaction was completed for 2 hours, quenched by adding a saturated solution of ammonium chloride, extracted with ethyl acetate (5ml×3), dried over anhydrous magnesium sulfate, filtered, and concentrated. Ethyl acetate / petroleum ether=1:10 The product obtained by column chromatography has a yield of 88% and a corresponding selectivity of 68%.
Embodiment 3
[0041] The catalytic product 3a (R 2 = Me,R 1 =Ph) Preparation
[0042] 5mL reaction flask, add copper acetate monohydrate (0.005mmol) and ligand L3 (0.01mmol), N 2 For protection, add 1 mL of dry toluene, stir and coordinate at room temperature for 3 hours, dissolve the substrate γ-phenyl β, γ-unsaturated α-keto acid methyl ester (0.25 mmol) in 1 mL of dry toluene and add dropwise to the reaction system and stir for 5 minute. Diethylzinc (0.375 mmol, 1.0 M in n-hexane) was slowly added dropwise. The system was reacted at -30°C, and the reaction was monitored by TLC. After 2 hours of reaction, a saturated solution of ammonium chloride was added to quench, extracted with ethyl acetate (5ml×3), dried over anhydrous magnesium sulfate, filtered, and concentrated. Ethyl acetate / petroleum ether=1:10 The product obtained by column chromatography has a yield of 99% and a corresponding selectivity of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com