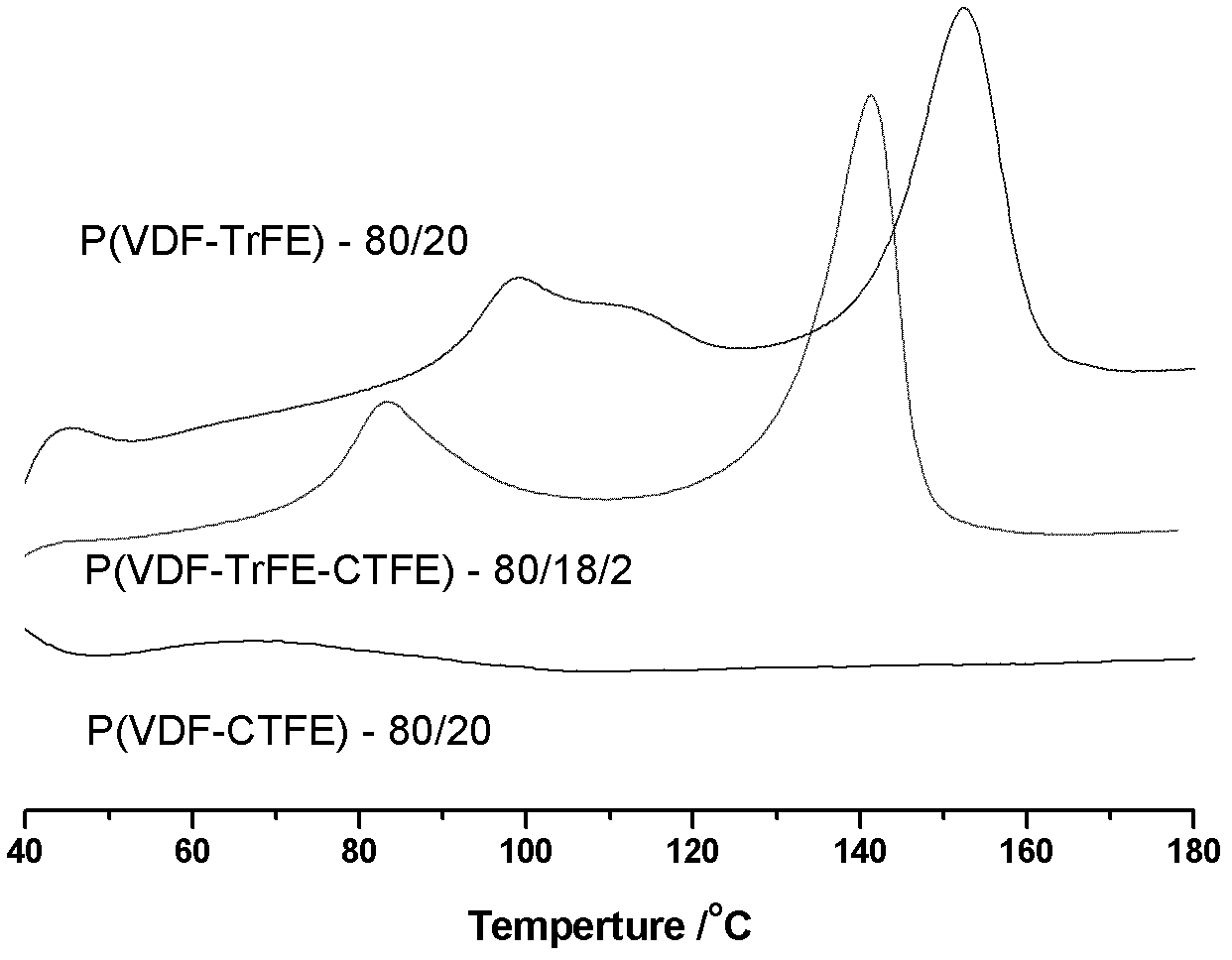
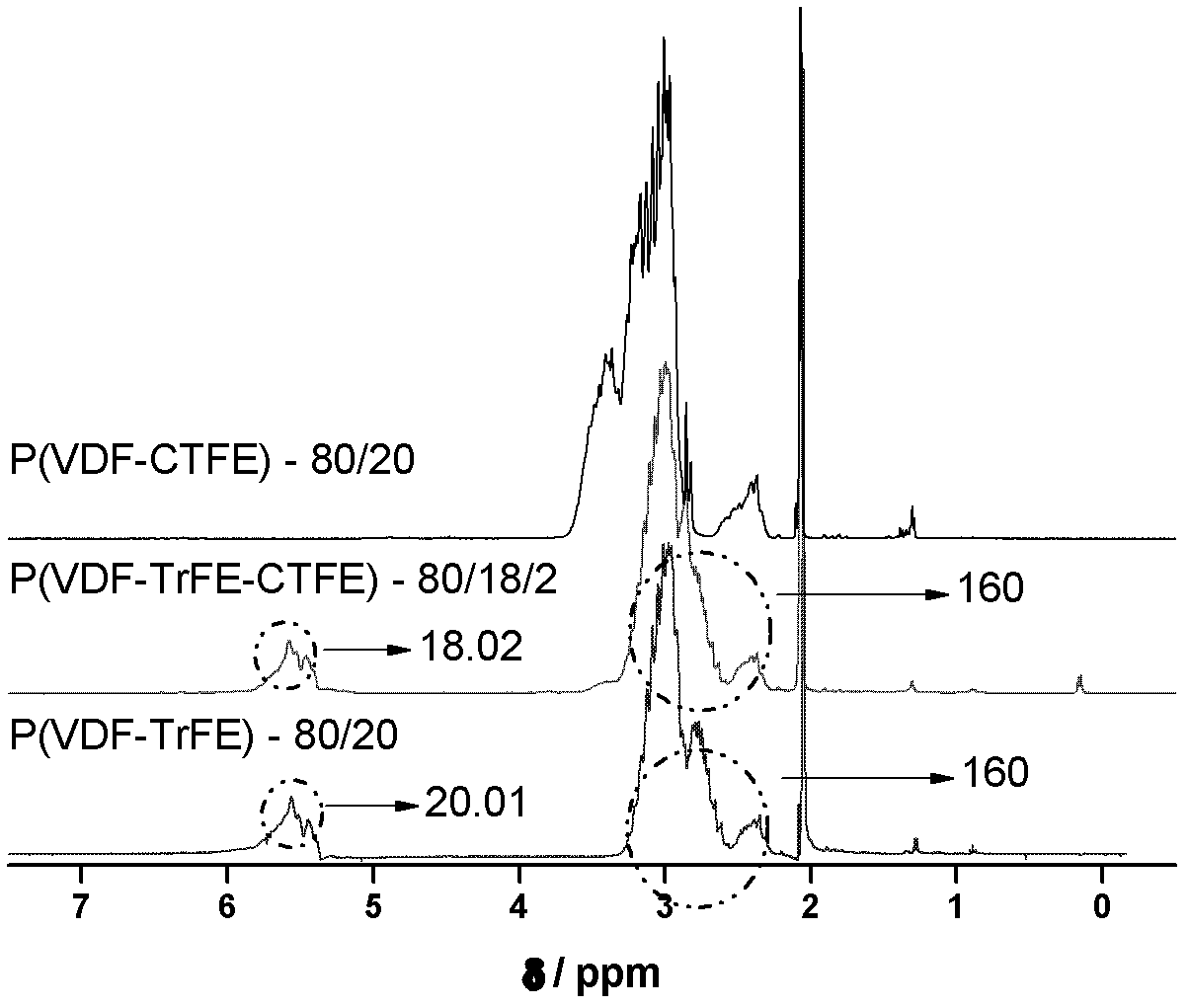
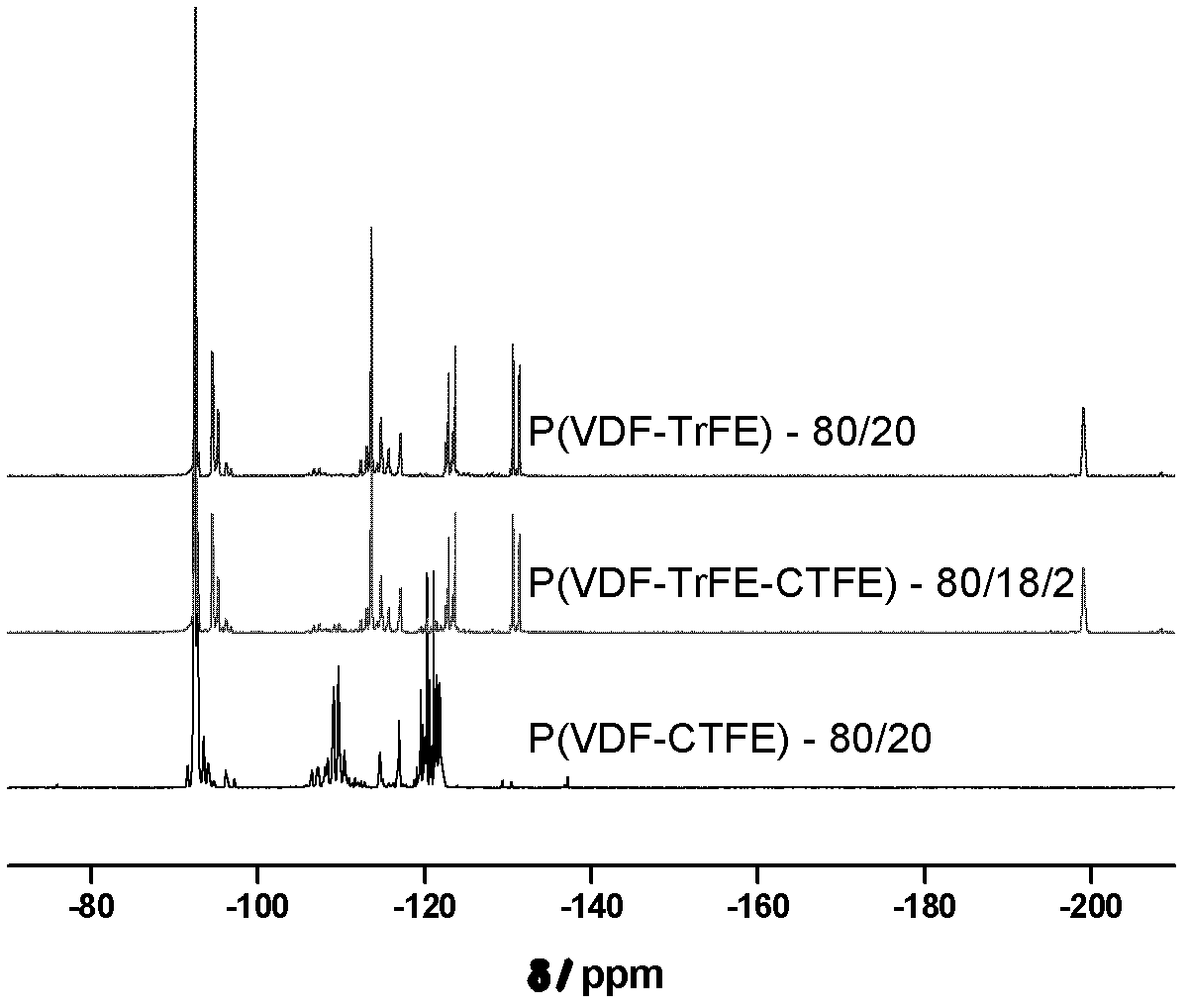
Method for preparing poly(vinylidene fluoride-trichloroethylene) or poly(vinylidene fluoride-chlorotrifluoroethylene-trichloroethylene)
A technology of chlorotrifluoroethylene and vinylidene fluoride, which is applied in the field of hydrogenation reduction reaction, can solve the problems of fluoropolymer elimination and degradation, copper salt is difficult to remove, etc., and achieves low cost, friendly to human body and environment, simple and easy to control method operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A method for preparing poly(vinylidene fluoride-chlorotrifluoroethylene-trifluoroethylene) by reduction of poly(vinylidene fluoride-chlorotrifluoroethylene) comprises the following steps:
[0020] Step 1. In the three-necked flask, add poly(vinylidene fluoride-trifluoroethylene) P(VDF-CTFE), the molar composition is VDF / CTFE=80:20, and at the same time add solvent, poly(vinylidene fluoride-trifluoroethylene) The mass ratio of chlorofluoroethylene) P(VDF-CTFE) to solvent is 4.5:100. After the poly(vinylidene fluoride-trifluorochloroethylene)P(VDF-CTFE) is fully dissolved, a reducing agent is added, and the reducing agent and poly (Vinylidene fluoride-chlorotrifluoroethylene)P(VDF-CTFE)The molar ratio of Cl atoms is 3.4:1, after the reductant is dissolved by magnetic stirring, continue to stir and react at room temperature for 20 hours;
[0021] Described solvent is tetrahydrofuran;
[0022] The reducing agent is LiAlH 4 ;
[0023] Step 2: Pour the final solution obtai...
Embodiment 2
[0027] A method for preparing poly(vinylidene fluoride-chlorotrifluoroethylene-trifluoroethylene) by reduction of poly(vinylidene fluoride-chlorotrifluoroethylene) comprises the following steps:
[0028]Step 1. In the three-necked flask, add poly(vinylidene fluoride-trifluoroethylene) P(VDF-CTFE), the molar composition is VDF / CTFE=80:20, and at the same time add solvent, poly(vinylidene fluoride-trifluoroethylene) The mass ratio of chlorofluoroethylene) P(VDF-CTFE) to solvent is 3.6:100. After the poly(vinylidene fluoride-trifluorochloroethylene)P(VDF-CTFE) is fully dissolved, a reducing agent is added, and the reducing agent and poly (Vinylidene fluoride-chlorotrifluoroethylene)P(VDF-CTFE)The molar ratio of Cl atoms is 2.7:1, after the reductant is dissolved by magnetic stirring, continue to stir and react at room temperature for 20 hours;
[0029] Described solvent is dimethyl sulfoxide;
[0030] The reducing agent is LiAlH 4 ;
[0031] Step 2: Pour the final solution obt...
Embodiment 3
[0034] A method for preparing poly(vinylidene fluoride-chlorotrifluoroethylene-trifluoroethylene) by reduction of poly(vinylidene fluoride-chlorotrifluoroethylene) comprises the following steps:
[0035] Step 1. In the three-necked flask, add poly(vinylidene fluoride-trifluoroethylene) P(VDF-CTFE), the molar composition is VDF / CTFE=80:20, and at the same time add solvent, poly(vinylidene fluoride-trifluoroethylene) The mass ratio of chlorofluoroethylene) P(VDF-CTFE) to solvent is 4.5:100, after poly(vinylidene fluoride-trifluorochloroethylene)P(VDF-CTFE) is fully dissolved, add reducing agent, reducing agent and poly (Vinylidene fluoride-chlorotrifluoroethylene)P(VDF-CTFE)The molar ratio of Cl atoms is 2.2:1, after the reductant is dissolved by magnetic stirring, continue to stir and react at room temperature for 20 hours;
[0036] Described solvent is tetrahydrofuran;
[0037] The reducing agent is NaBH 4 ;
[0038] Step 2: Pour the final solution obtained in step 1 into a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com