Synthesis of novel chiral polyester containing binaphthyl and application thereof to molecular recognition
A molecular recognition and binaphthyl technology, which can be used in the preparation of ethers by ester reaction, chemical instruments and methods, preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
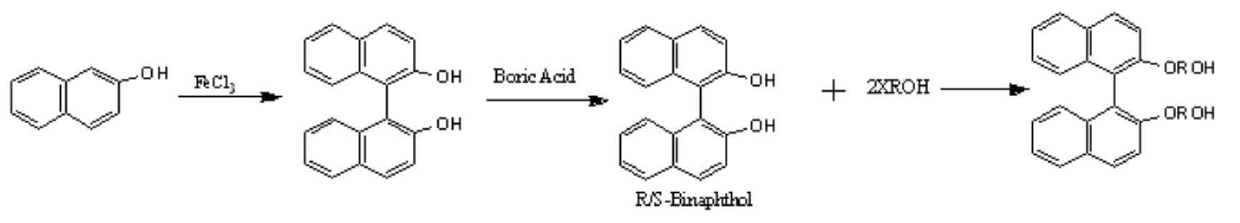
[0014] 2-Naphthol is reacted under ferric chloride to produce binaphthol, and then resolved to obtain R / S binaphthol. The resolved binaphthol is reacted with a halogenated alcohol to prepare a chiral diol containing a binaphthyl group.
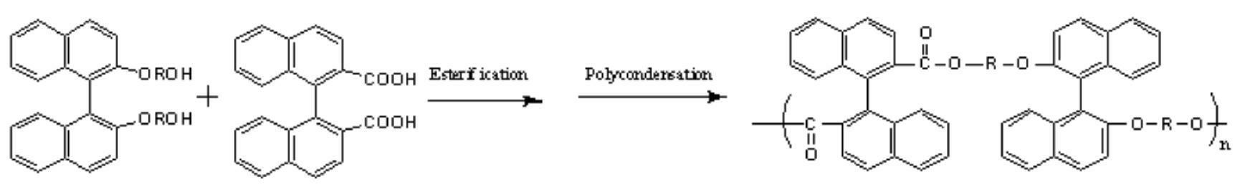
[0015] The prepared chiral diol containing binaphthyl group was reacted with diacid, and the chiral polyester was prepared through esterification and polycondensation reaction, and finally its structure was characterized by infrared and nuclear magnetic resonance. The reaction formula is as follows:
[0016]
[0017]
Embodiment 2
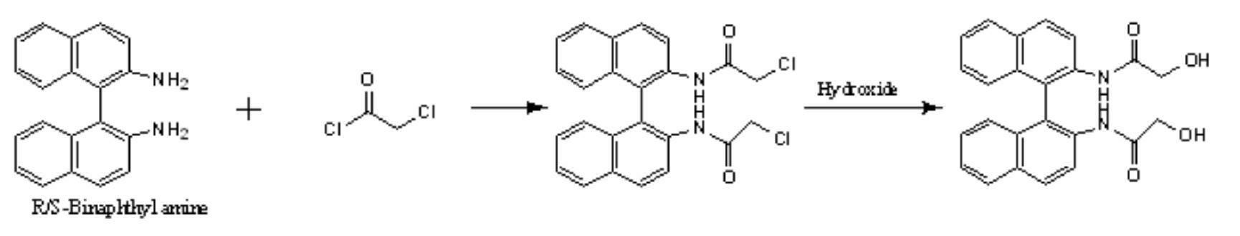
[0019] Using 2-naphthylamine as raw material, binaphthylamine is first synthesized, and then split to obtain chiral binaphthylamine. The resolved binaphthylamine is reacted with chloryl chloride, and then hydrolyzed to obtain binaphthyl-containing chiral diol.
[0020] The prepared chiral diol containing binaphthyl is reacted with diacid to prepare chiral polyester through esterification and polycondensation. Its structure was characterized by infrared and NMR. The detailed reaction formula is shown below.
[0021]
[0022]
Embodiment 3
[0024] Makes synthetic polyester into 1.0×10 -5 mol / L tetrahydrofuran solution, the chiral small molecule guest is also prepared as 0.1mol / L tetrahydrofuran solution, wherein mandelic acid is acetonitrile solution. For each measurement, the guest solution was pipetted with a pipette gun and added to the polymer solution to form a series of solutions, and the fluorescence intensity was measured after standing at room temperature for 3-4 hours.
[0025] The molecular recognition behavior of chiral polyesters for binaphthols was studied by UV-vis spectrometric titration. Chiral polyester host concentration 2×10 -6 mol / L, added binaphthol or binaphthylamine guest concentration 1×10 -6 mol / L, use phenol / tetrachloroethane (volume ratio 2:3) mixture as solvent, fix the concentration of chiral polyester, gradually increase the volume of the guest, and make the ultraviolet absorption spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com