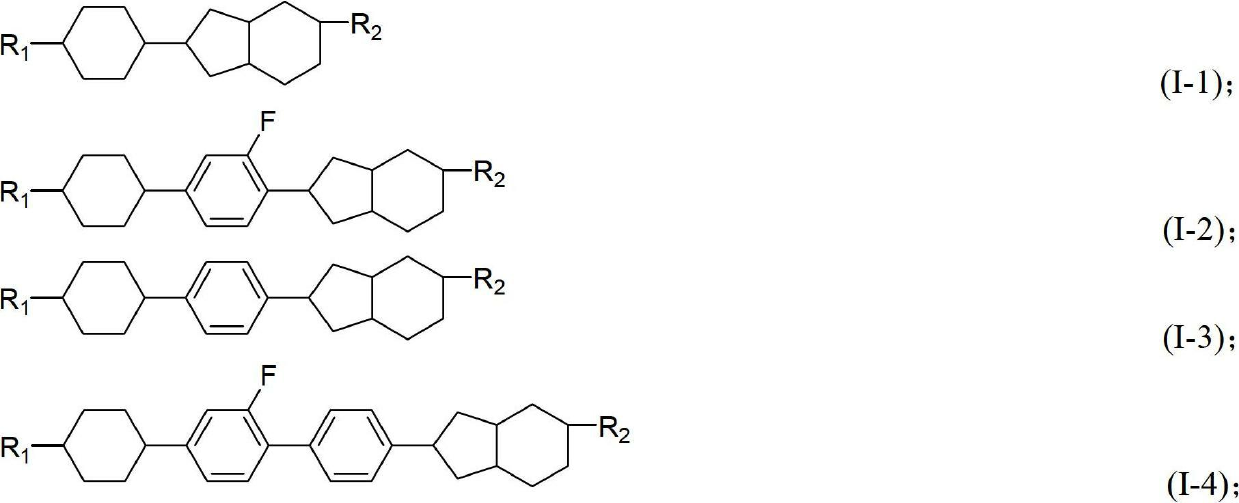
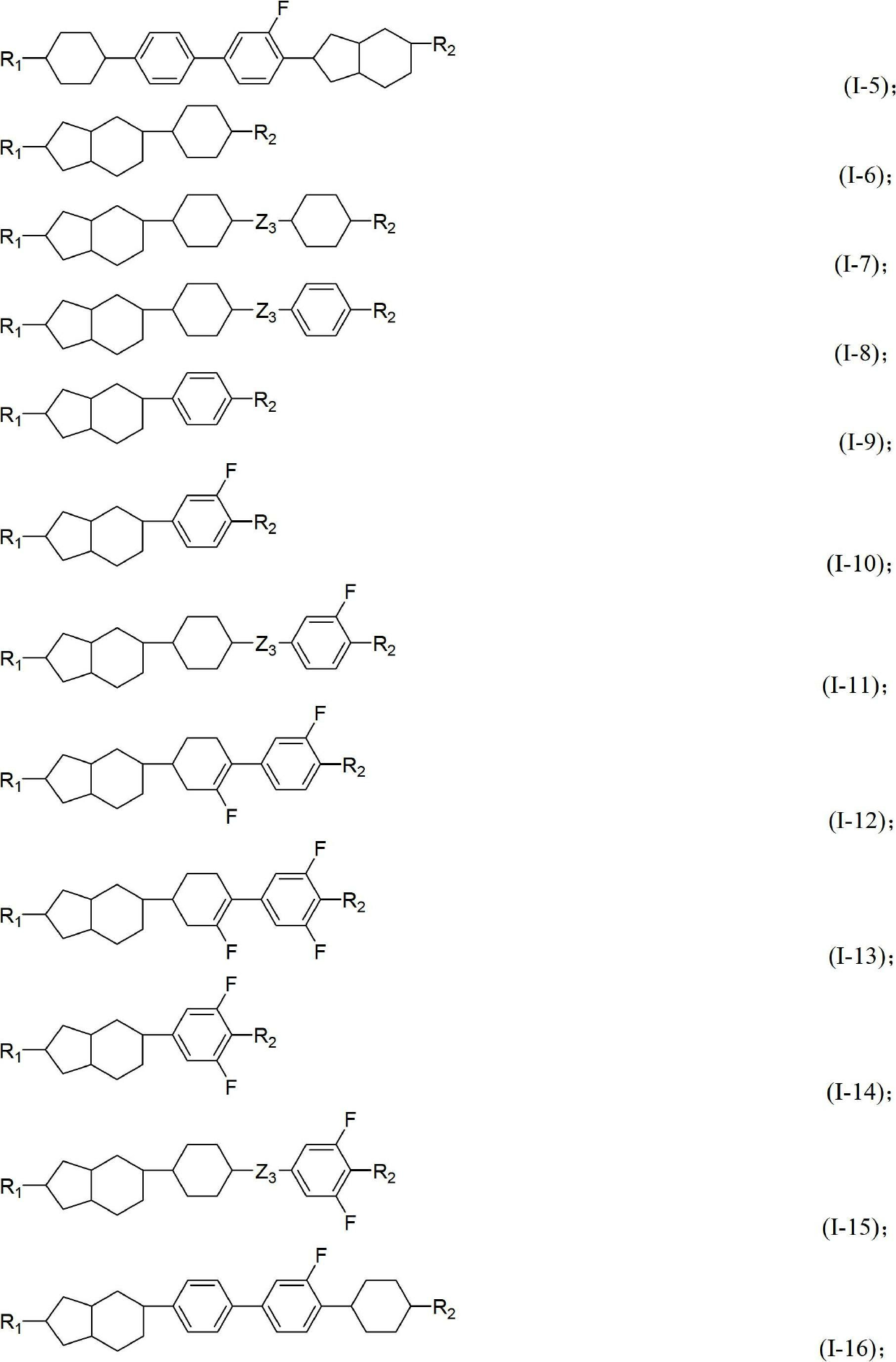
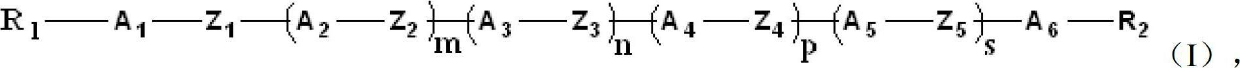Liquid crystal compound containing saturated indene rings and composition thereof
A compound, free technology, applied in liquid crystal materials, organic chemistry, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The synthetic route of the prepared compound 1IdCUF is as follows:
[0053]
[0054] Its specific preparation process is as follows:
[0055]
[0056] Among them, bicyclic monopropanone can be synthesized by methods known in the prior art (refer to US4307112 for its synthesis method).
[0057]
[0058] 1) Synthesis of Compound 1IdCUF-04
[0059]
[0060] Add 71g of methyl bromide triphenylphosphine salt, 300mL of tetrahydrofuran (THF) into a 1L three-necked flask, and add 2 Cooled to -5°C with ice-brine under protection, added 22 g of potassium n-butoxide, reacted for 30 min after the addition, and obtained an orange suspension. 50 mL of a THF solution of 60 g of monoethylene glycol-protected saturated indanone was added dropwise, and after the addition was completed, it was naturally raised to room temperature overnight at -5°C to 0°C. The reaction solution was transferred into a one-necked bottle, the solvent was spin-dried, and the residue was washed w...
Embodiment 2
[0074] The synthetic route of the prepared compound 1IdCGF is as follows:
[0075]
[0076] Its specific preparation process is as follows:
[0077] Compound 1IdCUF-02 was prepared in the same way as steps 1) to 3) in Example 1, and then 1IdCGF was obtained through the following synthesis steps.
[0078]
[0079]4) Synthesis of Compound 1IdCGF-01
[0080]
[0081] Using the intermediate 1IdCUF-02 for the preparation of 1IdCUF, 1IdCGF-01 was synthesized according to the method for synthesizing 1IdCUF-01 above, replacing 44.6 g of 5-(4 -Bromocyclohexyl)-1,2,3-trifluorobromobenzene. 31.4 g of 1IdCGF-01 can be prepared with a GC purity of 99.2% and a yield of 63%.
[0082] 5) Synthesis of Compound 1IdCGF
[0083] According to the method of synthesizing 1IdCUF, 1IdCGF-01 was hydrogenated and reduced, and then recrystallized and purified to obtain 18.87 g of the target product 1IdCGF with a GC purity of 99.7% and a yield of 59.8%.
[0084] 1 H NMR (CDCl 3 , 300MHz), ...
Embodiment 3
[0086] The synthetic route of the prepared compound 3IdZGF is as follows:
[0087]
[0088] Its specific preparation process can refer to the known methods in the prior art (the synthesis method refers to US5621147), as follows:
[0089]
[0090] 1) Synthesis of compound 3IdZGF-08
[0091]
[0092] Add bromopropane triphenylphosphine salt 270.7g, THF 1.2L, N 2 Cooled to -5°C with ice-brine under protection, added 80 g of potassium n-butoxide, reacted for 30 min after the addition, and obtained an orange suspension. 300 mL of a THF solution of 115 g of monoethylene glycol-protected saturated indanone was added dropwise, and after the addition was completed, it was naturally raised to room temperature overnight at -5°C to 0°C. The reaction solution was transferred into a one-necked bottle, the solvent was spin-dried, and the residue was washed with 200 mL of petroleum ether, then filtered with suction, and the filter cake was washed with petroleum ether several times....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com