L-amlodipine benzene sulfonate tablet and preparation process thereof
A kind of technology of levamlodipine besylate and levamlodipine besylate, applied in the field of levamlodipine besylate tablets and preparation technology thereof, can solve the problems of cumbersome types of auxiliary materials, complicated procedures, high cost of tablets, etc. The effect of high dissolution performance, simple preparation process and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
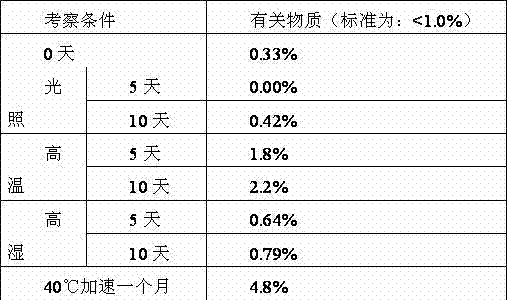
Examples
Embodiment 1
[0062] Embodiment 1, the screening of levamlodipine besylate tablet prescription
[0063] ①Dry powder compression
[0064] In the early trial production process, it was considered that the raw materials and auxiliary materials were evenly mixed and then directly pressed into tablets, but due to the poor fluidity of the powder, it could not fall into the hopper naturally. Therefore, the prescription process is selected as wet granulation followed by tablet compression.
[0065] ②Selection of adhesive
[0066] In the selection process of the binder, adding 5% hypromellose solution as the binder, the disintegration time of the prepared tablet is greatly prolonged, ranging from 10 to 12 minutes, which is likely to affect the quality of the finished product. Dissolution. Afterwards, 15% alcohol was used as a wetting agent for granulation, and the obtained tablets were bright and white, with excellent friability and disintegration. Therefore, 15% alcohol was used as a wetting ...
Embodiment 2
[0075] Embodiment 2, one of preparation technology of levamlodipine besylate tablet
[0076] Each 10,000 tablets of levamlodipine besylate contains:
[0077] Levoamlodipine Besylate 34.66g
[0078] Microcrystalline Cellulose 350g
[0080] Hypromellose 90g
[0082] The preparation method of the levamlodipine besylate tablet described in the present embodiment comprises the following steps:
[0083] (1) Weigh 34.66g of levamlodipine besylate, 350g of microcrystalline cellulose, 350g of calcium sulfate, 90g of hydroxypropyl cellulose, and 8g of magnesium stearate;
[0084] (2) Pass microcrystalline cellulose, calcium sulfate and hydroxypropyl cellulose through a 60-mesh sieve, and mix well;
[0085] (3) Levoamlodipine besylate passes through a 80-mesh sieve, diffuses in grades in the mixture obtained in step (2), puts it into a wet mixing granulator, and stirs for 10 to 20 minutes;
[0086] (4) Add an appropri...
Embodiment 3
[0097] Embodiment 3, the second of preparation process of levamlodipine besylate tablet
[0098] Each 10,000 tablets of levamlodipine besylate contains
[0099] Levoamlodipine Besylate 34.66g
[0100] Microcrystalline Cellulose 280g
[0101] Calcium sulfate 280g
[0102] Hypromellose 72g
[0103] Magnesium Stearate 6.4g
[0104] The preparation method of the levamlodipine besylate tablet described in the present embodiment comprises the following steps:
[0105] (1) Weigh 34.66g of levamlodipine besylate, 280g of microcrystalline cellulose, 280g of calcium sulfate, 72g of hydroxypropyl cellulose, and 6.4g of magnesium stearate;
[0106](2) Pass microcrystalline cellulose, calcium sulfate and hydroxypropyl cellulose through a 60-mesh sieve, and mix well;
[0107] (3) Levoamlodipine besylate passes through a 80-mesh sieve, diffuses in grades in the mixture obtained in step (2), puts it into a wet mixing granulator, and stirs for 10 to 20 minutes;
[0108] (4) Add an app...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com