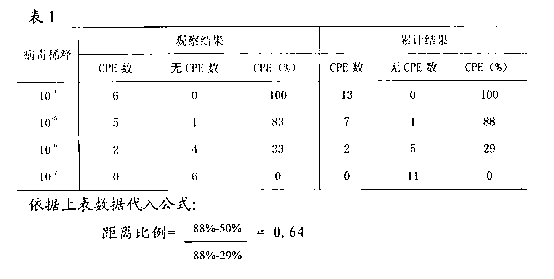
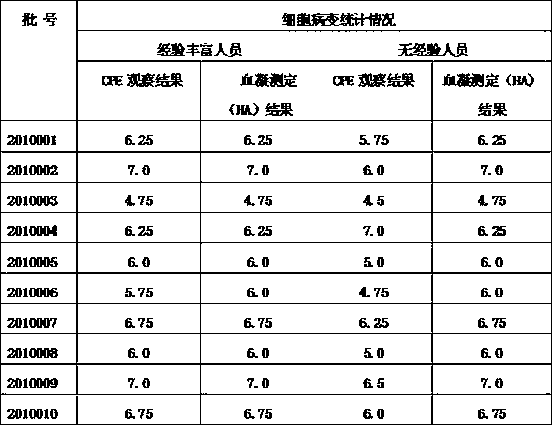
Determining method of content of antigen virus of inactivated vaccine of recombinant bird flu cellgen
A technique of inactivated vaccine and determination method, which is applied in the field of determination of antigen and virus content of recombinant avian influenza cell-derived inactivated vaccine, can solve the problems of allergic reaction, subjective error, unobservation, etc., and achieves the achievement of highlighting technological progress and reducing errors. , Benchmark reliable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The invention will be further described below in conjunction with embodiment.
[0019] Detection process: steps of resuscitating and culturing MDCK cells; virus solution dilution, inoculation, red blood cell agglutination; calculation of TCID 50 step;
[0020] (1) Preparation of MDCK cells:
[0021] The sampling density is 2.0×10 6 1.5ml of frozen MDCK cells were placed in a 37°C water bath, shaken to dissolve for 3 minutes, centrifuged at 1000rmp / min for 5 minutes, discarded the supernatant, added 15ml of cell culture medium, and transferred to a 75cm 2 culture flask at 37°C, 5% CO 2 In the incubator, after culturing for 72 hours, the cells were digested with 0.25% EDTA-trypsin, and then diluted with cell nutrient solution (10% fetal bovine serum + DMEM) to 4.0×10 5 The MDCK cell suspension was plated on a 96-well cell plate at a cell density of 40,000 / well (0.1ml / well), and placed at 37°C, 5% CO 2 In the incubator, cultivate for 24 hours to form a dense monolayer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com