Novel 1,2,3-triazole derivative of chitosan and preparation method thereof
A technology of chitosan and derivatives, applied in botany equipment and methods, chemicals for biological control, biocides, etc., can solve the problems of gaps in antibacterial activity and achieve improved antibacterial activity and good solubility properties, improving the effect of poor solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 derivative 1
[0025] 1.58 grams of chitosan azide derivatives with a molecular weight of 230,000, 1.60 grams of propargyl benzoate, 0.70 grams of ascorbic acid, and 0.50 grams of copper sulfate were added to 50 mL of water, reacted at 60 ° C for 12 hours, cooled to room temperature, and the reactants were pumped out. Filter, wash with absolute ethanol, and dry at 60° C. to obtain a brown powder, which is chitosan derivative 1. The structural formula is shown in formula I (R is phenyl).
[0026] The preparation of the chitosan azido derivatives can be found in the following documents: WEIZHONG YUAN, ZHENGDA ZHAO, SHUYING GU, JIE REN. Synthesis, Characterization, and Properties of Amphiphilic Chitosan Copolymers with Mixed Side Chains by Click Chemistry. Journal of Polymer Science: Part A: Polymer Chemistry, Vol.48, 3476 3486.
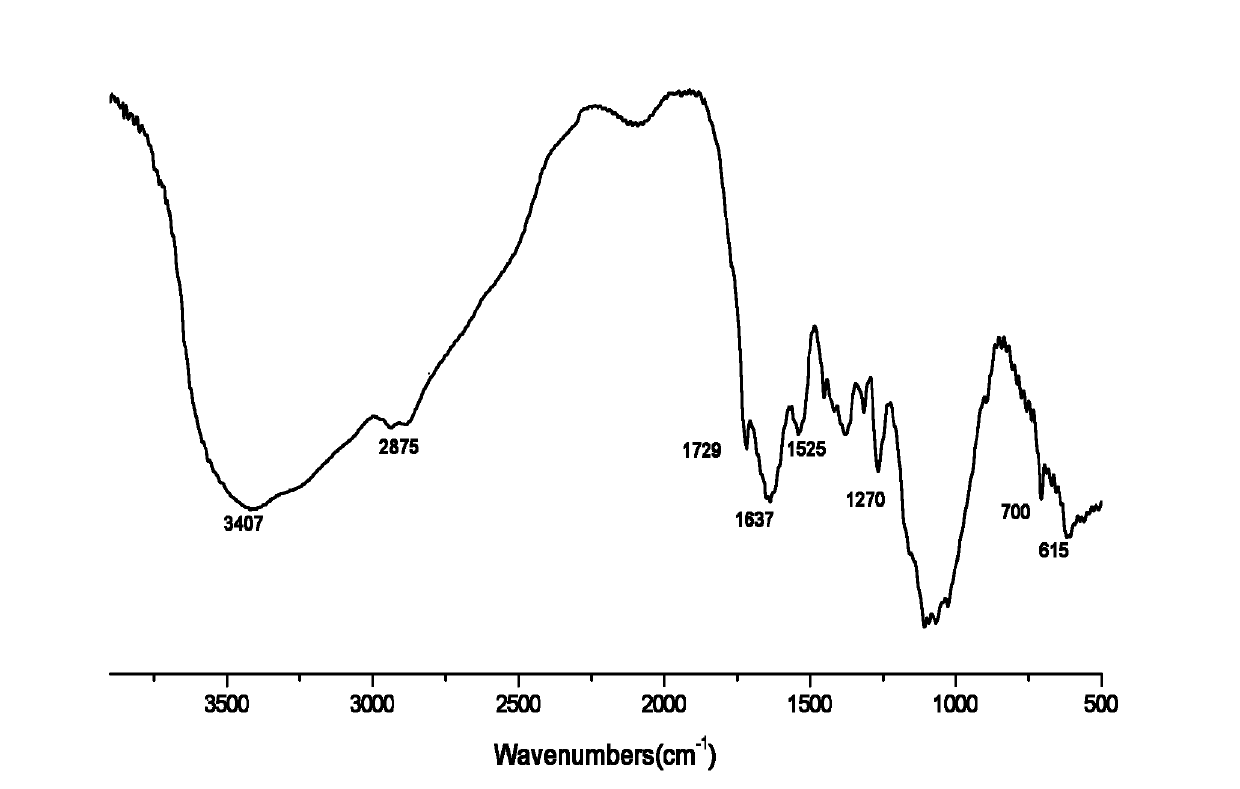
[0027] Infrared spectrum shows: the infrared spectrum of chitosan derivative 1 ( image 3 ) and the infrared s...
Embodiment 2
[0028] The preparation of embodiment 2 derivative 2
[0029] 1.58 grams of chitosan azide derivatives with a molecular weight of 230,000, 1.61 grams of propargyl benzoate, 0.70 grams of ascorbic acid, and 0.50 grams of copper sulfate were added to 50 mL of water, reacted at 60 ° C for 12 hours, cooled to room temperature, and the reactants were pumped out. Filter, wash with absolute ethanol, and dry at 60° C. to obtain a brown powder, which is chitosan derivative 1. The structural formula is shown in formula I (R is 3-pyridyl).
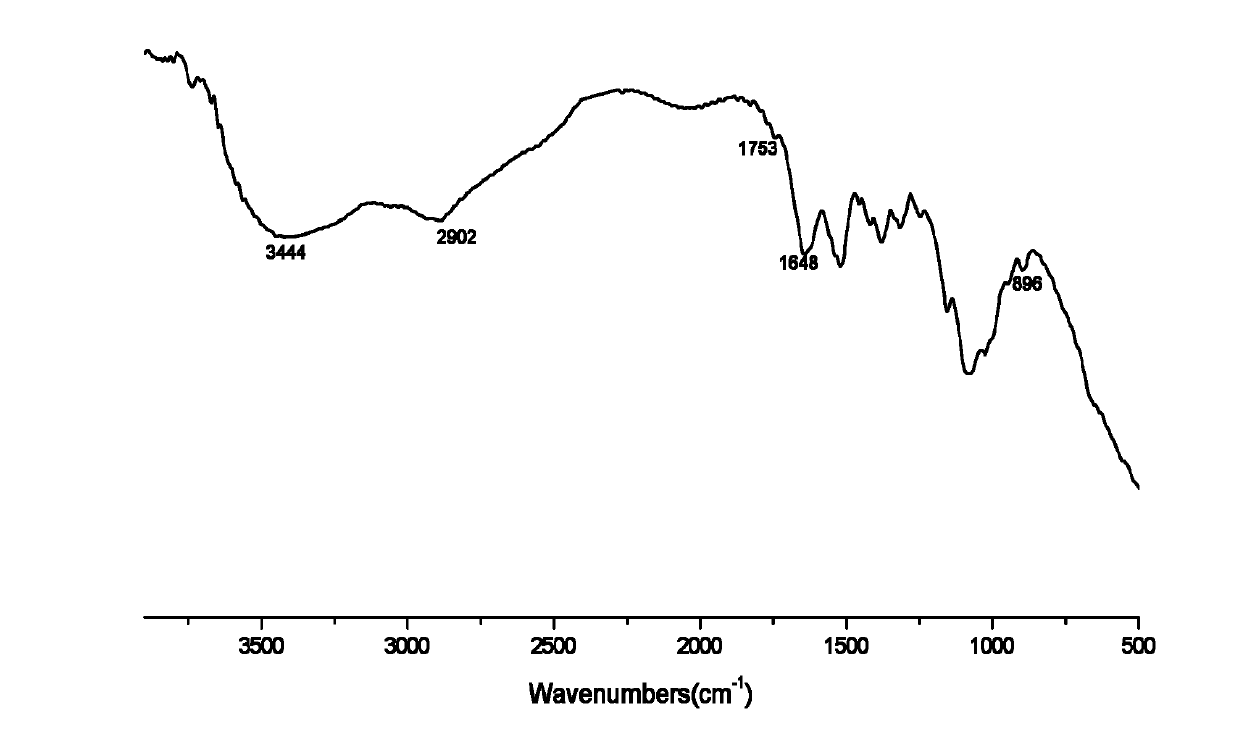
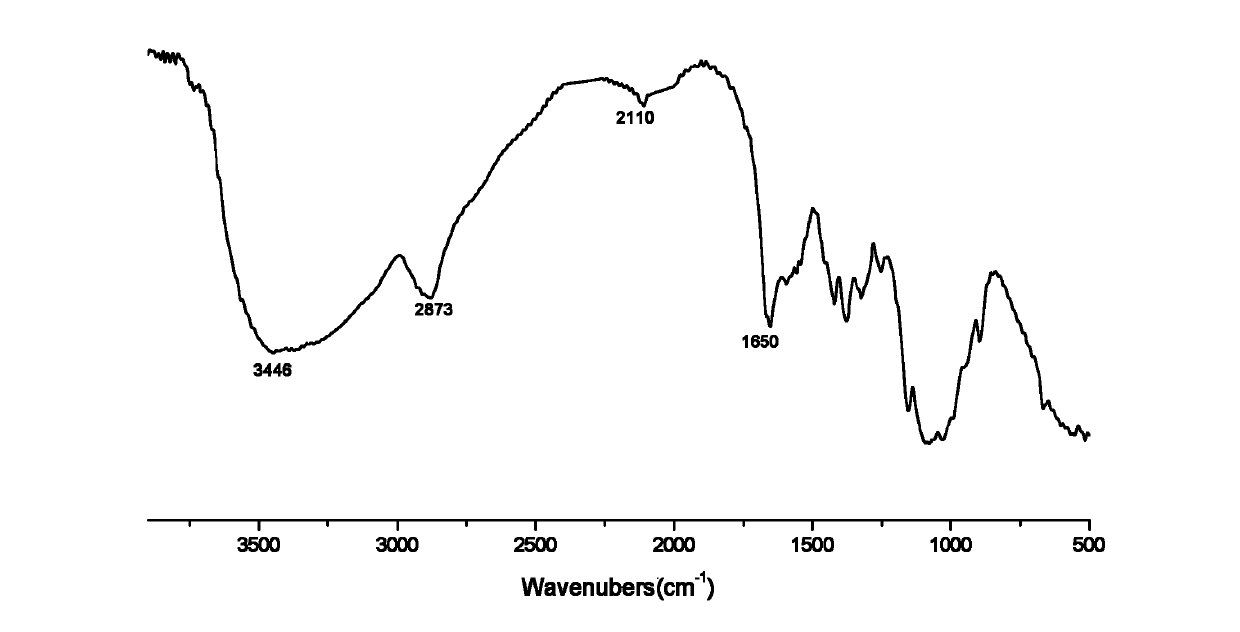
[0030] Infrared spectrum shows: the infrared spectrum of chitosan derivative 2 ( Figure 4 ) and the infrared spectrum of chitosan azide derivatives ( figure 2 ) compared to that at 2110cm -1 The absorption peak of the N≡N absorption peak disappears, and the characteristic absorption peak of the ester group appears at 1726, 1645cm -1 The characteristic absorption peak at C=N, 736, 611cm -1 It is the characteristic absorption of the pyridine ring,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com