Drug delivery system of small interfering RNA drug and preparation
A technology for small interfering nucleic acids and drugs, which is applied in the field of drug delivery systems and preparations of small interfering RNAs, can solve the problems of low drug loading, low siRNA encapsulation rate, and difficulty in meeting clinical applications, and achieves simple preparation methods and encapsulation. The effect of high rate and drug loading, low potential toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1, the preparation of siRNA delivery system and preparation
[0061] siRNA-loaded nanoparticles were prepared by a double-emulsion method using amphiphilic block polymers and cationic lipids. The polyethylene glycol-polylactic acid or polyethylene glycol-poly(lactic acid glycolic acid) diblock / triblock copolymer used is the above-mentioned polymer PEG 550 -PLA 28600 、PEG 2000 -PLA 12500 、PEG 5000 -PLA 5000 、PEG 5000 -PLA 25000 、PEG 5000 -PLA 51000 、PEG 10000 -PLA 15000 、PEG 10000 -PLGA 10000(50 / 50) 、PEG 10000 -PLGA 50000(75 / 25) , PLA 6300 -PEG 1200 -PLA 6300 , PLA 4800 -PEG 5000 -PLA 4800 , PLA 4800 -PEG 10000 -PLA 4800 (Subscripts are molecular weight and ratio). The cationic lipids used were the above-mentioned BHEM-Chol, DOTAP, and DOTMA, respectively. The siRNA drug delivery system with different properties is prepared by changing the quality of the added cationic lipid, the quality of the added siRNA, the type of polymer used, an...
Embodiment 2
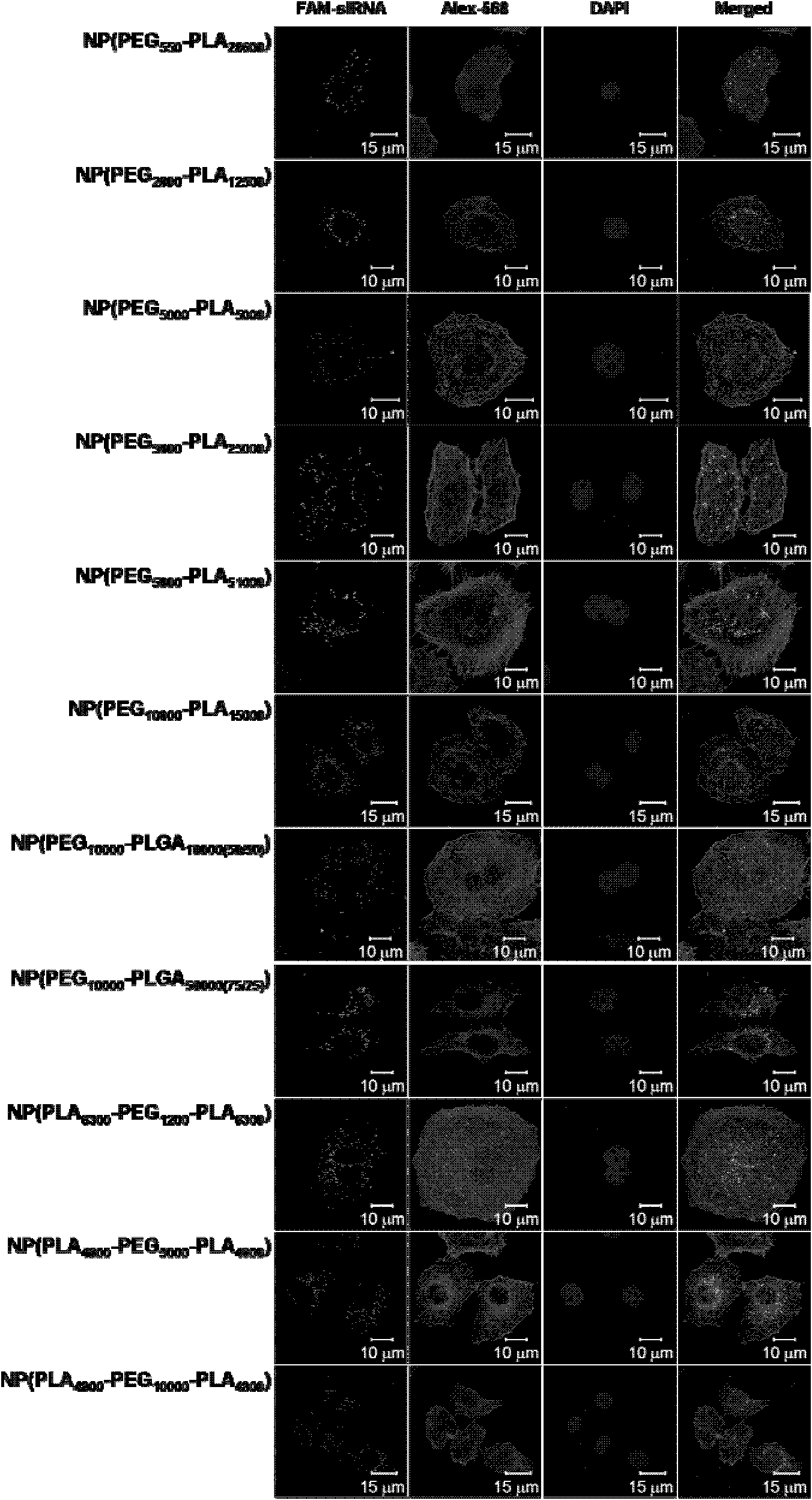
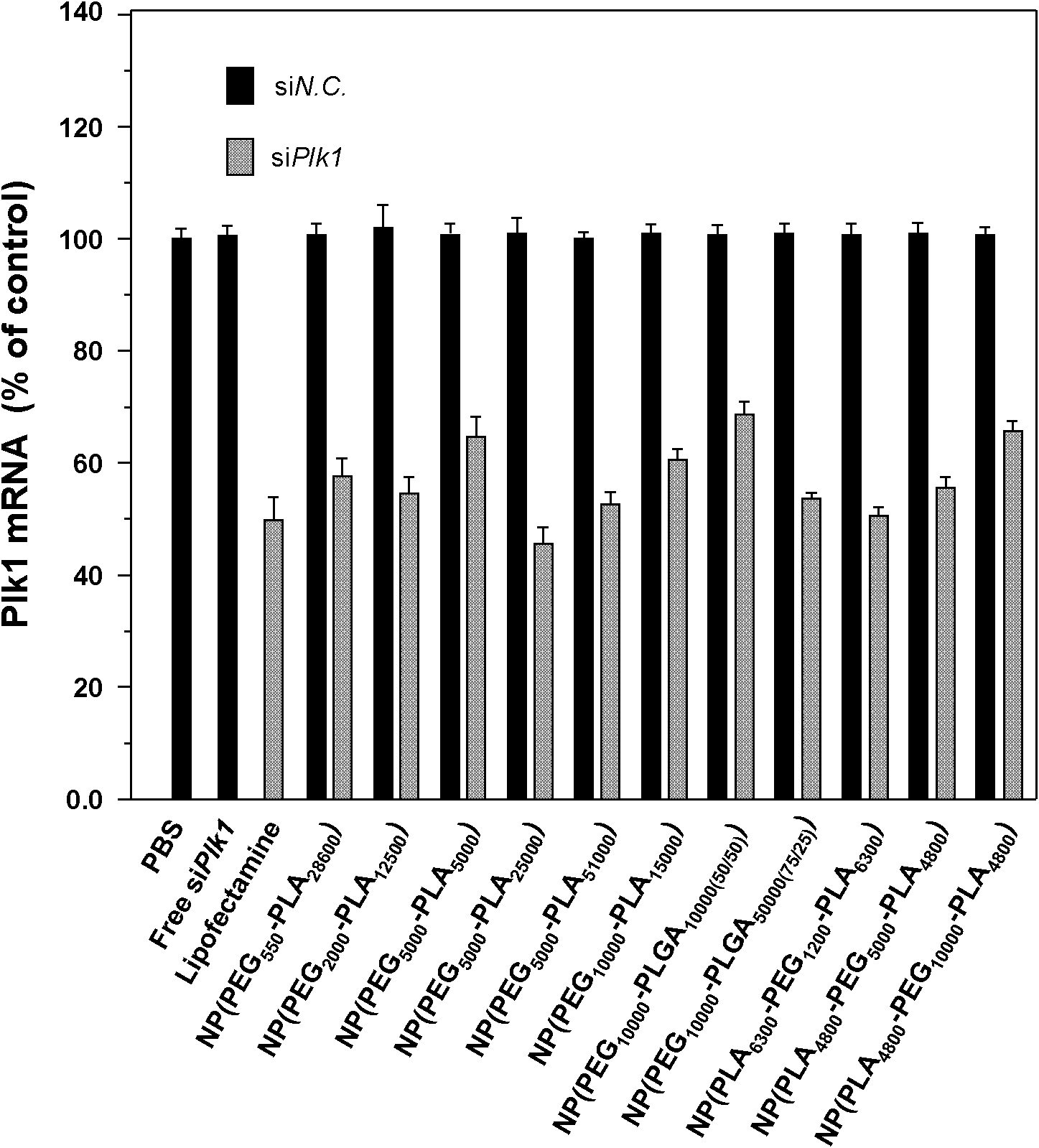
[0109] Embodiment 2, the evaluation of the effect of this drug delivery system at the cellular level
[0110] According to Example 1, when siRNA / cationic lipid / polymer=0.2 / 1.0 / 25.0, the obtained siRNA drug delivery system has higher siRNA encapsulation efficiency and drug loading capacity. Therefore, this example is used to illustrate the biological effect of this drug delivery system. The polymer used is PEG 550 -PLA 28600 、PEG 2000 -PLA 12500 、PEG 5000 -PLA 5000 、PEG 5000 -PLA 25000 、PEG 5000 -PLA 51000 、PEG 10000 -PLA 15000 、PEG 10000 -PLGA 10000(50 / 50) 、PEG 10000 -PLGA 50000(75 / 25) , PLA 6300 -PEG 1200 -PLA 6300 , PLA 4800 -PEG 5000 -PLA 4800 , PLA 4800 -PEG 10000 -PLA 4800 , the cationic lipid used is BHEM-Chol.
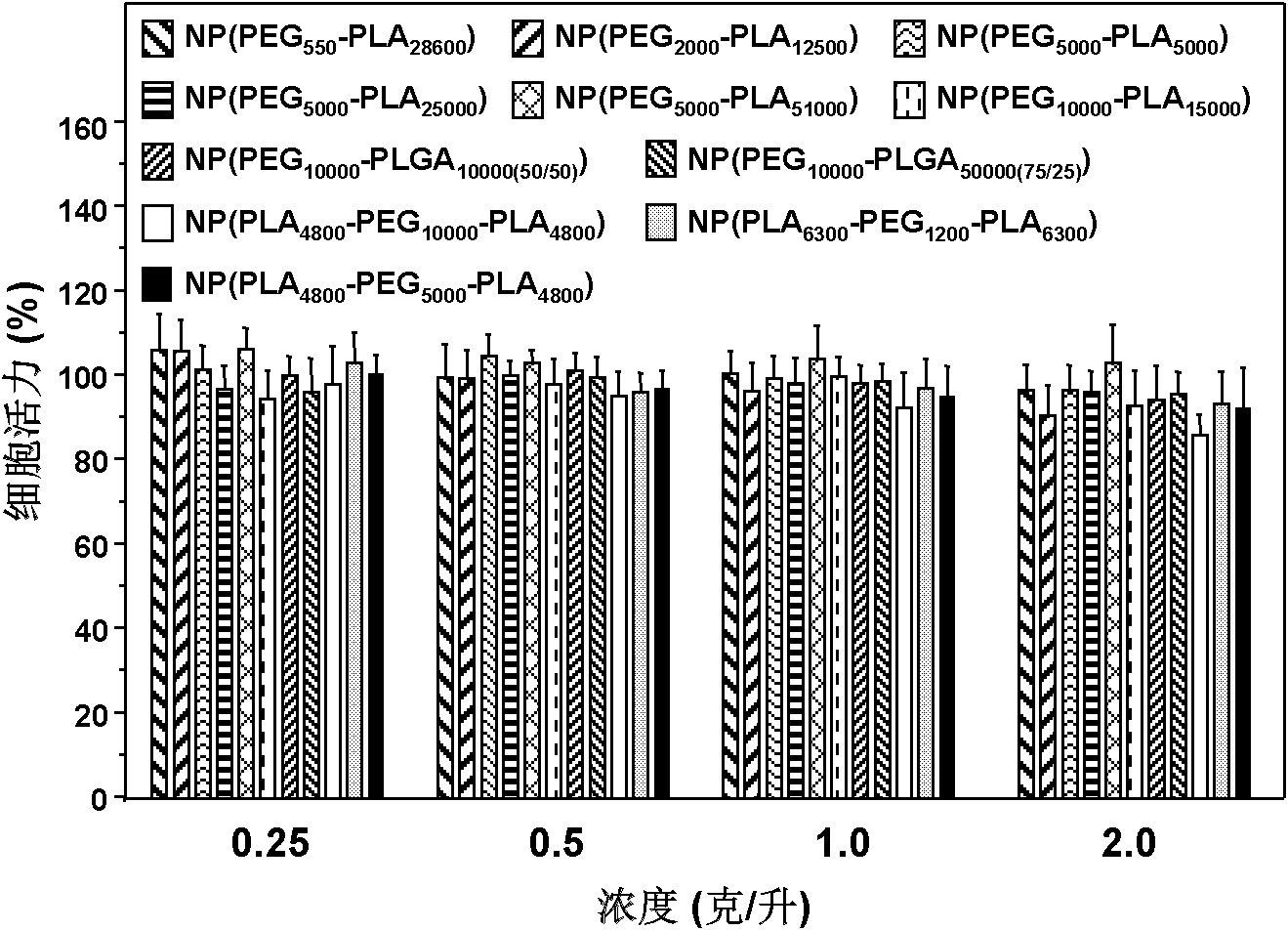
[0111] 1. Biocompatibility evaluation of nanoparticles prepared from different polymers
[0112] Nanoparticles not loaded with siRNA were prepared by the method described in Example 1. The toxicity of nanoparticles to human liver cance...
Embodiment 3
[0166] Embodiment 3, the biological effect evaluation of this drug delivery system at animal level
[0167] It can be seen from the experiment at the cell level that when the method described in Example 1 is used to prepare nanoparticles loaded with siRNA, when the ratio of each component is siRNA / cationic lipid / polymer=0.2 / 1.0 / 25.0, the cationic lipid used is BHEM-Chol, the polymer used is PEG 5000 -PLA 25000 When the prepared nanoparticles loaded with siRNA can effectively enter the cells, and can significantly silence the expression of the target gene, this formula is used to prepare the nanoparticles loaded with siRNA NP (PEG 5000 -PLA 25000 ) as an example to study the biological effects of this drug delivery system at the animal level.
[0168] 1. Inhibition of firefly luciferase expression by tumor cells implanted in liver orthotopically implanted with siLuci nanoparticles
[0169] Luciferase is firefly luciferase, which can catalyze the emission of visible light th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com