Method for preparing refined strong brine by removing potassium from strong brine
A technology of concentrated brine and potassium ions, which is applied in the field of preparing refined concentrated brine, can solve the problems of refined concentrated brine that have not been seen yet, and achieve the effects of low K+ content, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The first step is to use an ion exchange column filled with modified zeolite to remove potassium by adsorption of raw concentrated brine
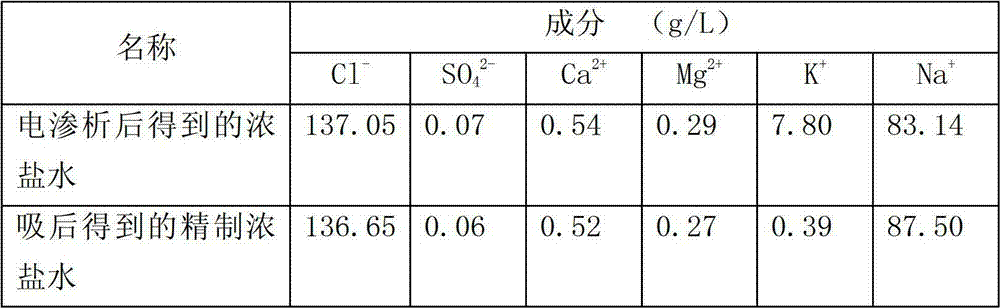
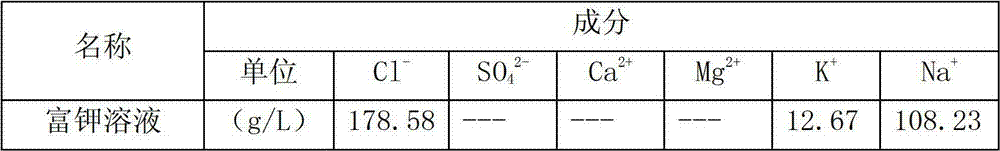
[0020] At 25°C, the concentrated brine obtained after electrodialysis of the raw material is passed into an ion exchange column filled with modified zeolite for adsorption reaction. The ion exchange column filled with modified zeolite is separated by 9 single ion exchange columns. It consists of 3 operating units, each single ion exchange column is composed of 800g of modified zeolite packed in a column with a diameter of 36mm and a height of 1000mm, and 3 ion exchange columns are set as an operating unit. Using the simulated moving bed technology to absorb the concentrated brine of the raw material, the potassium ions in the concentrated brine of the raw material exchange completely with the sodium ions on the modified zeolite. One operating unit, each operating unit is fed with 3L of concentrated brine, the adsorption flow rate of ...
Embodiment 2
[0028] The first step is to use an ion exchange column filled with modified zeolite to remove potassium by adsorption of raw concentrated brine
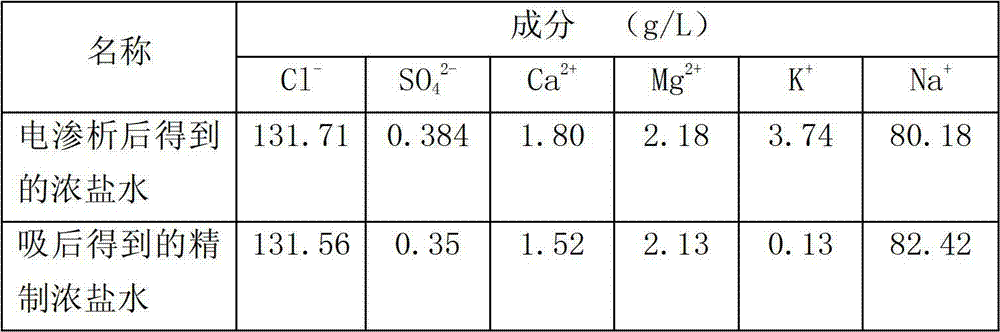
[0029] At 15°C, feed the raw brine into the ion-exchange column filled with modified zeolite for adsorption reaction. The ion-exchange column filled with modified zeolite is composed of 12 single ion-exchange columns divided into 4 operating units. Each single ion exchange column is composed of 800g of modified zeolite packed in a column with a diameter of 36mm and a height of 1000mm, and 3 ion exchange columns are set as an operation unit. Use the simulated moving bed technology to absorb the concentrated brine of the raw material, and the potassium ions in the concentrated brine of the raw material exchange completely with the sodium ions on the modified zeolite. One operating unit, each operating unit is fed with 8L of concentrated brine, the adsorption flow rate of the raw concentrated brine, that is, the empty tower flow rate, i...
Embodiment 3
[0037] The first step is to use an ion exchange column filled with modified zeolite to remove potassium by adsorption of raw concentrated brine
[0038] At 40°C, the raw brine is passed into the ion exchange column packed with modified zeolite for adsorption reaction. The ion exchange column packed with modified zeolite is composed of 6 single ion exchange columns divided into 2 operating units. Each single ion exchange column is composed of 800g of modified zeolite packed in a column with a diameter of 36mm and a height of 1000mm, and 3 ion exchange columns are set as an operation unit. Use the simulated moving bed technology to absorb the concentrated brine of the raw material, and the potassium ions in the concentrated brine of the raw material exchange completely with the sodium ions on the modified zeolite. One operating unit, each operating unit is fed with 21L of concentrated brine, the adsorption flow rate of the raw concentrated brine, that is, the empty tower flow ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com