Preparation method of ethyl 6-oxo-8-chloro-caprylate
A technology of ethyl chlorooctanoate and ethyl hexanoate, which is applied in the field of preparation of lipoic acid intermediates, can solve the problems of increased wastewater volume and low cooling efficiency, and achieve reduced wastewater volume, high cooling efficiency, and good industrial application value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
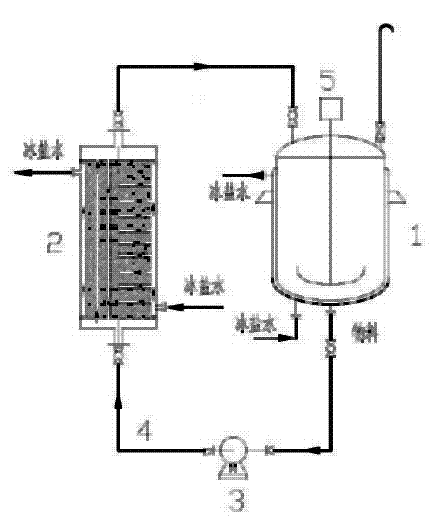
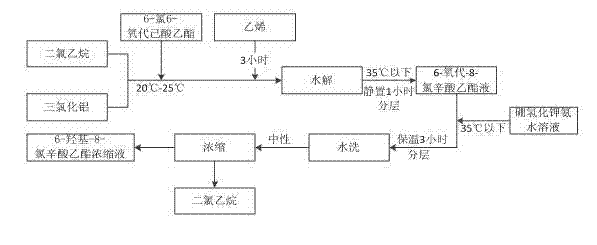
[0032] 4000kg of water into 10M 3 In the hydrolysis kettle, ice salt water is passed into the jacket of the hydrolysis kettle. The hydrolysis kettle is equipped with a circulation pump and a circulation pipeline. The circulation pipeline is connected to a graphite condenser. Brine, the material is cooled and then recycled back to the hydrolysis tank. Use a jacket and a graphite condenser to circulate and cool the water in the hydrolysis tank to below 10°C for later use.
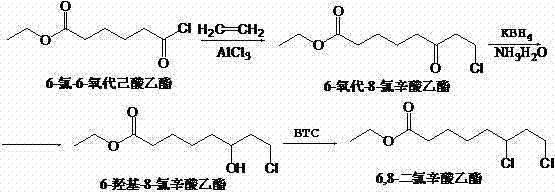
[0033] Add 1280Kg of dichloroethane into a 1500L reaction kettle, add 450Kg of aluminum trichloride, stir, cool down to 10°C-18°C with an ice-salt water jacket, add dropwise 360kg of ethyl 6-chloro-6-oxohexanoate, and Control the temperature at 20°C to 25°C, and pass through ethylene for 3 hours to obtain the addition reaction solution. Put the reaction liquid into the spare 4000kg water in the hydrolysis kettle, stir it, and then enter the graphite condenser through the circulation pump to cool it, then re...
Embodiment 2
[0036] 4000kg of water into 10M 3 In the hydrolysis kettle, ice salt water is passed into the jacket of the hydrolysis kettle. The hydrolysis kettle is equipped with a circulation pump and a circulation pipeline. The circulation pipeline is connected to a graphite condenser. Brine, the material is cooled and then recycled back to the hydrolysis tank. Cool with jacket ice brine and graphite condenser ice brine, cool down to below 10°C by circulation, and set aside.
[0037] Add 1280Kg of dichloroethane into a 1500L reaction kettle, add 450Kg of aluminum trichloride, stir, cool down to 10°C-18°C with an ice-salt water jacket, add dropwise 360kg of ethyl 6-chloro-6-oxohexanoate, and Control the temperature at 20°C to 25°C, and pass through ethylene for 3 hours to obtain the addition reaction solution. Put the reaction liquid into the spare 4000kg water in the hydrolysis kettle, stir it, and then enter the graphite condenser through the circulation pump to cool it, then return i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com