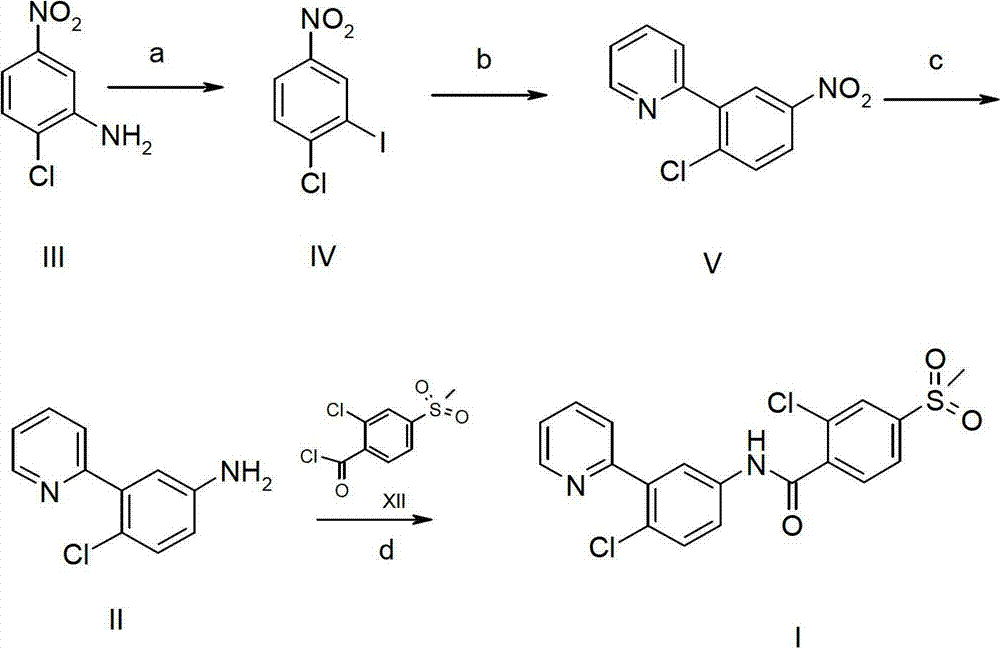
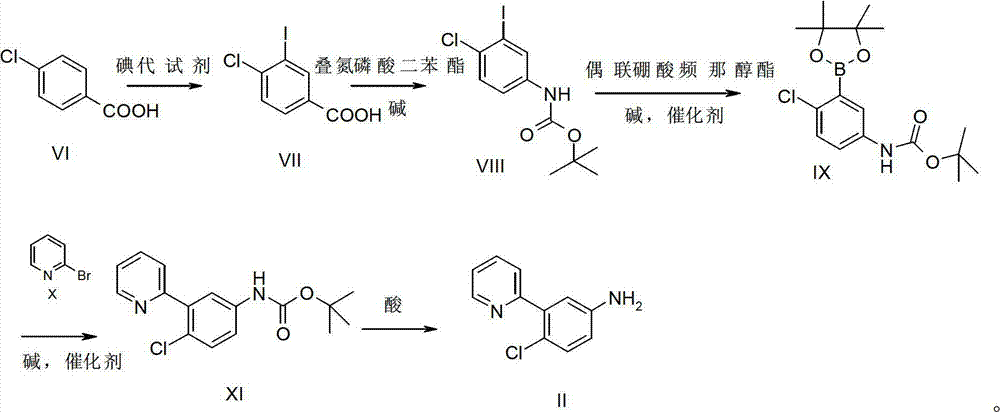
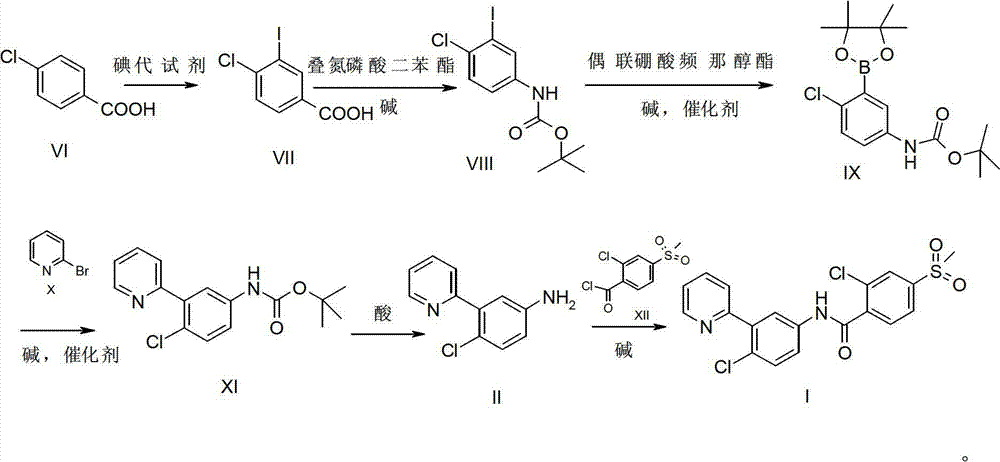
Preparation method of intermediate of antitumor drug GDC-0449 (vismodegib)
A technology of intermediates and compounds, which is applied in the field of synthesis of drugs and their intermediates, can solve the problems of not very mild reaction conditions, unstable large-scale production yields, and high raw material costs, and achieve cheap raw materials, high yields, and excellent reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of Intermediate II
[0024] Synthesis of compound VII:
[0025]
[0026] Add compound VI (313.1g, 2.0mol, 1.0eq.) in 1L four-neck flask, dissolve with 5L concentrated sulfuric acid, add NaIO 4 (188.2g, 0.9mol, 0.45eq.), when the solid is completely dissolved, slowly add KI (365g, 2.2mol, 1.1eq.) at room temperature, the reaction solution is dark purple, control the reaction temperature 25-30℃, After addition, react at room temperature for 2h, after the reaction is complete. The reaction solution was slowly poured into 20 kg of crushed ice, stirred, a large amount of solids precipitated, filtered, the filter cake was washed with water and petroleum ether, and dried to obtain 410 g of compound VII as a white solid, with a yield of 72.7%. 1 HNMR (400MHz, DMSO-d 6 )δ (ppm): 13.35 (bs, 1H), 8.38 (d, J=2.0Hz, 1H), 7.91 (dd, J=8.3, 2.0Hz, 1H), 7.69 (d, J=8.3Hz, 1H) .
[0027] Synthesis of Compound VIII:
[0028]
[0029] In a 1L four-neck flask, add com...
Embodiment 2
[0040] Synthesis of compound I:
[0041]
[0042] Add compound II (81g, 400mmol, 1.0eq.) in 2L four-neck flask, 800mLDCM, add triethylamine (Et 3 N) (60g, 600mmol, 1.5eq.), then slowly add compound XII (120g, 480mmol, 1.2eq.) in an ice bath, control the temperature at 0-5°C, complete the addition, react at room temperature for 30min, and the reaction ends. Recrystallization (PE / EA) yielded 165.3 g of a white solid with a yield of 99%. Purity: 99%. 1 H NMR (400MHz, CDCl3) δ (ppm): 9.58 (bs, 1H), 8.43 (d, J = 4.7Hz, 1H), 8.03 (dd, J = 2.6, 8.7Hz, 1H), 7.90 (d, J =1.6Hz, 1H), 7.67-7.78(m, 4H), 7.60(d, J=8.0Hz, 1H), 7.51(d, J=8.8Hz, 1H), 7.23-7.24(m, 1H), 3.01 (s, 3H).
Embodiment 3
[0044] Synthesis of compound VII:
[0045]
[0046] Add compound VI (313.1g, 2.0mol, 1.0eq.) in 1L four-neck flask, dissolve with 5L concentrated sulfuric acid, add NaIO 4 (104.5g, 0.49mol, 0.25eq.), when the solid is completely dissolved, slowly add KI (248.8g, 1.50mol, 0.75eq.) at room temperature, the reaction solution is black and purple, control the reaction temperature 25-40℃ , After addition, react at room temperature for 3h, after the reaction is complete. The reaction solution was slowly poured into 18 kg of crushed ice, stirred, a large amount of solids precipitated, filtered, the filter cake was washed with water and petroleum ether, and dried to obtain 400 g of compound VII as a white solid, with a yield of 70.9%. 1 HNMR (400MHz, DMSO-d 6 )δ (ppm): 13.35 (bs, 1H), 8.38 (d, J = 2.0Hz, 1H), 7.91 (dd, J = 8.3, 2.0Hz, 1H), 7.69 (d, J = 8.3Hz, 1H) .
[0047] Synthesis of Compound VIII:
[0048]
[0049] In a 1L four-necked flask, add compound VII (331.3g, 1.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com