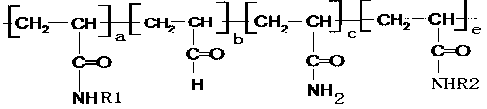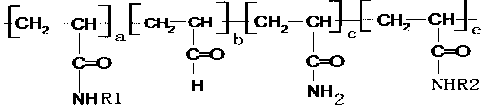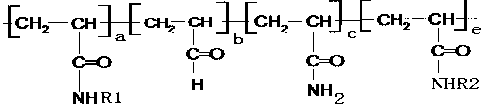Aldehydic hydrogen-based self-crosslinking polyacrylamide
A technology of polyacrylamide and aldehyde hydrogen, which is applied in the field of self-crosslinking polyacrylamide, can solve the problems of complex construction process, high construction cost, and many equipments, and achieve good profile control effect, convenient construction and safe use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 260Kg of acrylamide, 20Kg of N-methylolphenol carbonyl acrylamide, 20Kg of N-methylolacrylamide, and 700Kg of deionized water into the reaction kettle. Stir with nitrogen gas for 25 minutes, and then add an initiator. The initiator has a mass ratio of 1: 1 Potassium persulfate: sodium sulfite, initiate polymerization at 25°C for 6 hours, then react at 40°C for 55 minutes, dry and granulate to obtain self-crosslinked polyacrylamide reacted with aldehyde hydrogen.
[0023] In the reaction, R1 and R2 are mutually reactive groups. R1 is The terminal group, R2 is end group. The ratio of R1 to R2 is 1:1. The content of the monomers of R1 and R2 in the polymer was 1% by mass.
[0024]
Embodiment 2
[0026] Add 250Kg acrylamide, 25Kg N-methylol phenol carbonyl acrylamide, 25Kg N-methylol acrylamide, 750Kg deionized water into the reaction kettle, blow nitrogen gas for 30 minutes and stir well, then add initiator, the initiator is mass ratio 1: 1 Potassium persulfate: sodium sulfite, initiate polymerization reaction at 30°C for 7 hours, then react at 50°C for 60 minutes, dry and granulate to obtain self-crosslinked polyacrylamide reacted with aldehyde hydrogen.
[0027] In the reaction, R1 and R2 are mutually reactive groups. R1 is The terminal group, R2 is containing The terminal group; the ratio of R1 to R2 is 1:1. The content of monomers containing R1 and R2 in the polymer was 25% by mass.
Embodiment 3
[0029] Add 270Kg of acrylamide, 30Kg of N-methylol phenol carbonyl acrylamide, 30Kg of N-methylol acrylamide, and 800Kg of deionized water into the reaction kettle. Stir for 35 minutes with nitrogen gas, and then add the initiator. The initiator has a mass ratio of 1: 1 Potassium persulfate: sodium sulfite, initiate polymerization reaction at 30°C for 8 hours, then react at 60°C for 65 minutes, dry and granulate to obtain self-crosslinked polyacrylamide reacted with aldehyde hydrogen.
[0030] In the reaction, R1 and R2 are mutually reactive groups. R1 The terminal group, R2 is containing end group. The ratio of R1 to R2 is 1:1. The content of monomers containing R1 and R2 in the polymer is 50% by mass.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com