Applications of alliinase, and medicinal composition
A technology of alliinase and composition, which is applied in the direction of drug combination, medical preparation containing active ingredients, pharmaceutical formula, etc., to achieve the effect of expanding the application range and reducing the amount of homocysteine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
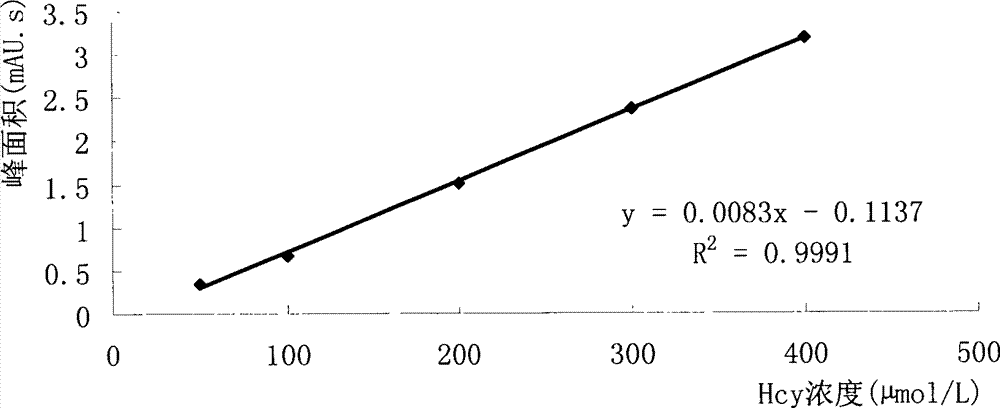
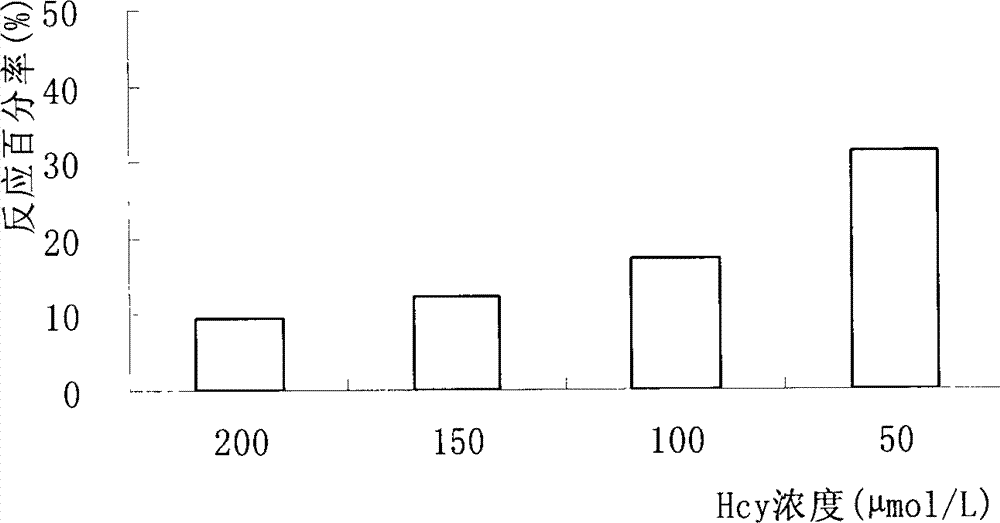
[0025] Example 1 Decomposition of alliinase to Hcy
[0026] 1. Decomposition of Hcy by alliinase in sucrose activity assay buffer
[0027] 1. Test method
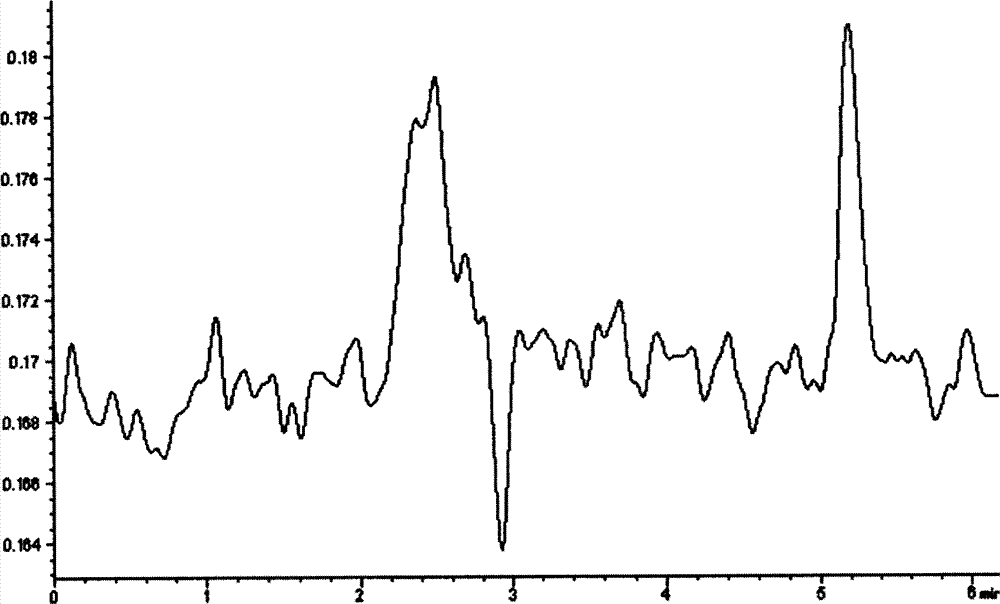
[0028] Prepare Hcy into 50, 100, 200, 300 and 400 μmol / L solutions with sucrose activity assay buffer, take 240 μl of each concentration, add 240 μl to alliinase solution prepared with sucrose activity assay buffer, and make alliin The unit of enzyme activity is 100u / ml, put it in a constant temperature water bath at 35°C for reaction immediately, take 240 μl of the above-mentioned reaction sample after 1 hour according to the sample processing method (references: Fan Xinping, Liu Dexiao, Shi Jiandang, etc., rHPLC quantitative analysis of plasma Hcy method Comparative research [J], Tianjin Medicine, 2003, 31 (9): 589-592), carry out HPLC operation (chromatographic column: C 18 Chromatographic column (3.9mm×150mm, 5μm); column temperature: 20°C; mobile phase: 0.1mol / lNaH 2 PO 4 -Na 2 HPO 4 Buffer (pH4.0, containing 3...
Embodiment 2
[0067] The therapeutic effect of embodiment 2 alliinase on rat Hhcy
[0068] 1. Test method
[0069] Test according to literature method (Ma Xuexing, Liu Wangming, Wu Baiming, etc., the influence of folic acid, vitamin B6, vitamin B12 on the high homocysteine level and arteriosclerosis caused by high methionine in rabbits [J]. Hypertension Journal, 2002, 10 (1): 84-86.): 24 rats, half male and half female, weighing about 200g, were randomly divided into 4 groups: negative control group, model group, positive control group, alliinase group, 6 rats in each group , and the experiment started after one week of routine feeding. Blood was collected from the orbital venous plexus after fasting for 12 hours before modeling to determine the plasma Hcy content. After the modeling started, except the negative control group, animals in other groups were given methionine 1.5g / kg body weight once a day by intragastric administration for 6 consecutive weeks. On the 35th day of modeling,...
Embodiment 3
[0086] Example 3 The preventive effect of alliinase on hyperhomocysteinemia
[0087] 1. Experimental animals: big-eared white rabbits, grade I, male and female, body weight 2.0-2.5kg.
[0088] 2. Determination method of plasma homocysteine concentration
[0089] Sample processing: Take 2-3 mL of fasting blood (anticoagulated with 0.1% EDTA), immediately cool it in an ice bath, and centrifuge within 30 minutes to collect plasma and store it at -20°C. Take 240 μL plasma as the measurement sample, add acetylcysteine (final concentration 0.5mmol L -1 ) as an internal control, with 3-butylphosphine (final concentration 60mmol L -1 ) at 4°C for 30 minutes to fully reduce the sulfhydryl group, 0.6mol L -1 Treat denatured protein with perchloric acid, centrifuge at 2000g for 10min, take 50μL of supernatant and add 1.55mol·L -1 NaOH 10μL, boric acid buffer 125μL (0.125mol L -1 , pH 9.5, containing EDTA 4mmol·L -1 ), 50μL SBD-F (1g L -1 ), in a water bath at 60°C for 60 min, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com