Allopurinol derivative and preparation method and application thereof
A compound, N-4- technology, applied in drug combination, bone diseases, organic chemistry, etc., to achieve the effect of inhibiting xanthine oxidase activity, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
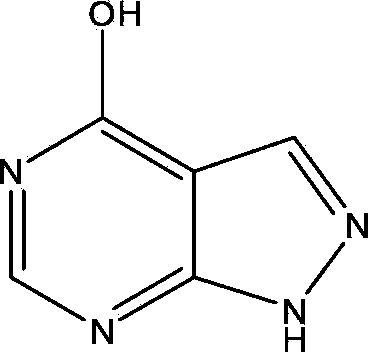
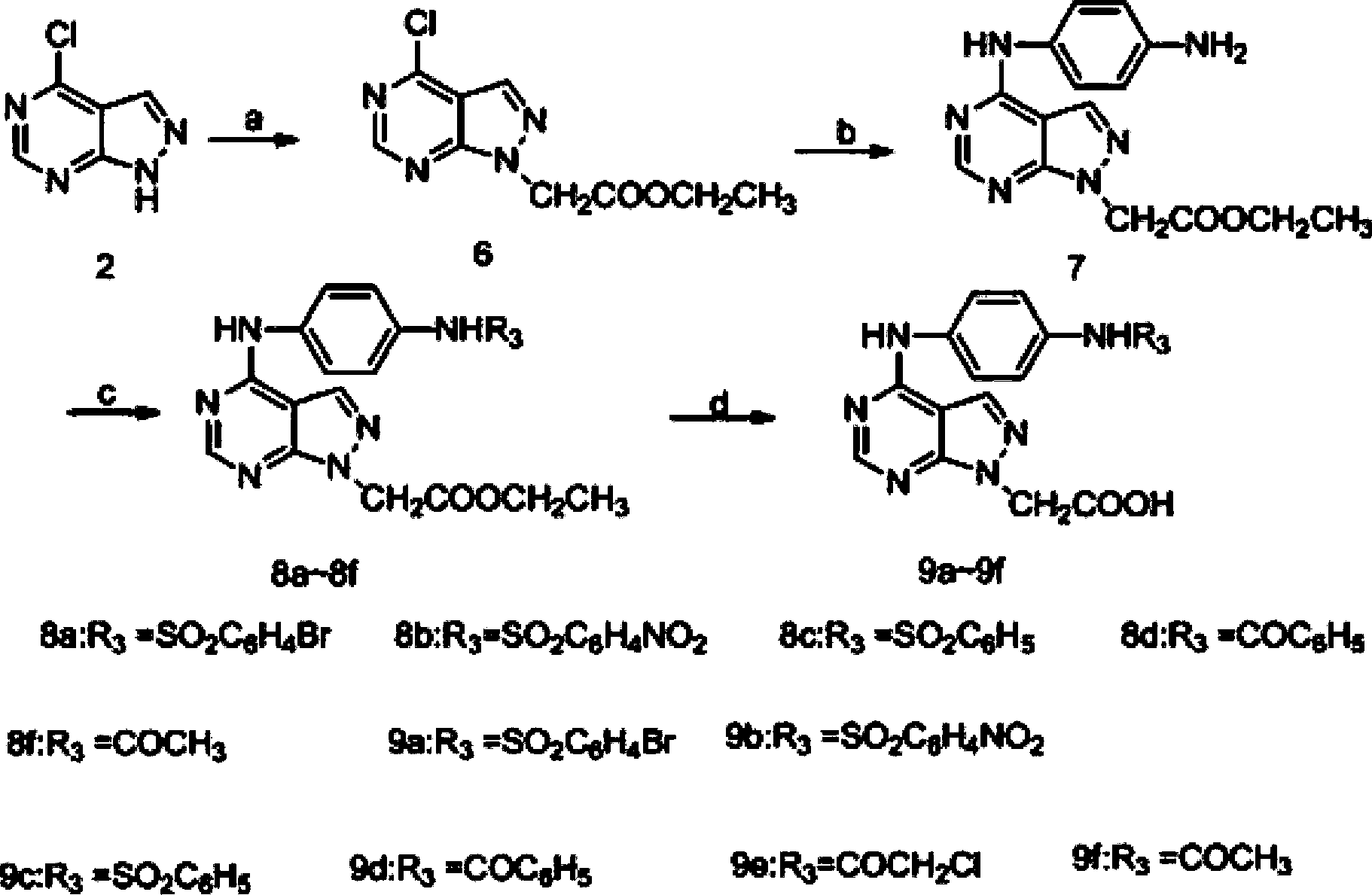
[0055] Example 1 Synthesis of (4-chloro-1H-pyrazol[3,4-d]pyrimidine-N-1-) ethyl acetate (compound 6 for short)
[0056] Slowly add TEA (1.78g, 17.62mmol) into 4-chloro-1H-pyrazolo[3,4-d]pyrimidine (0.8lg, 5.26mmol) dissolved in 20mL dry DMF, stir at room temperature for 30min, Ethyl bromoacetate (1.06 g, 6.39 mmol) dissolved in 5 mL of dry DMF was slowly added dropwise to the mixture and stirring was continued for 2 h. The reaction solution was detected by TLC and poured into 30mL of water after the reaction was complete. After adjusting the pH value to acidic with dilute hydrochloric acid, it was extracted with ethyl acetate (3×25mL). The organic layer was washed with saturated sodium chloride and concentrated to obtain flocs . The crude product was subjected to silica gel column chromatography and eluted with petroleum ether: ethyl acetate (6:1) to obtain white needle crystals (compound 6).
[0057] Compound 6: white crystal, 83~84℃ 1 H NMR (400MHz, DMSO-d 6 )δ:8.91(s,1H...
Embodiment 2
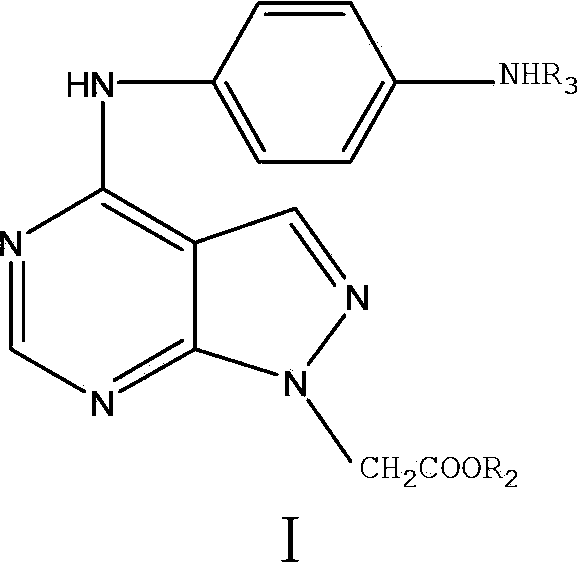
[0058] Example 2 Synthesis of (N-4-(4-aminophenyl)amino-1H-pyrazol[3,4-d]pyrimidine-N-1-)ethyl acetate (compound 7 for short)
[0059] Compound 6 (1.73g, 7.21mmol) was dissolved in 25mL of acetonitrile, then p-phenylenediamine (0.78g, 7.21mmol) was slowly added to the mixture, the reactant was heated to 80°C and stirred for 3h, detected by TLC to Total response. The reaction solution was concentrated to obtain a solid crude product, which was chromatographed on a silica gel column and eluted with petroleum ether: acetone (2:1) plus a few drops of triethylamine to obtain brown flaky crystals (compound 7).
[0060] Compound 7: Gray solid, 179.8~181.9°C 1 H NMR (400MHz, DMSO-d 6 )δ: 9.76 (br, 1H, NH), 8.27 (s, 2H, CH), 7.37 (s, 2H, ArH), 6.62 (s, 2H, ArH), 5.02~5.18 (m, 4H), 4.14 ( dd, J=7.04Hz, J=14.16Hz, 2H, CH 2 ), 1.19(t, J=7.08Hz, 3H, CH 3 ); 13 C NMR (100MHz, DMSO-d 6 )167.80,155.73,154.30,153.61,132.52,127.41,123.34,113.94,100.76,61.18,47.80,13.94;IR(KBr,ν,cm -1 ):...
Embodiment 3
[0061] The synthesis of embodiment 3 compound 8a~8c
[0062] Compound 7 (0.36g, 1.15mmol) was dissolved in 10mL THF, and NaH (0.069g, 2.88mmol) was slowly added to the mixture under ice conditions, stirred for 30min on ice, and then dissolved in 3mL Bromobenzenesulfonyl chloride (0.34g, 1.34mmol) in THF was slowly added dropwise to the reaction solution. After the dropwise addition was completed, stirring was continued for 1 h, and finally moved to room temperature and stirred overnight. The reaction solution was poured into 15 mL of water, adjusted to a pH of about 3 with dilute hydrochloric acid, extracted with dichloromethane (3×15 mL), the organic layer was washed with saturated sodium chloride, and concentrated to obtain a solid. The crude product was subjected to silica gel column chromatography, eluting with petroleum ether: acetone (2:1), to obtain 8a as a white solid.
[0063] Compound 7 (0.36g, 1.15mmol) was dissolved in 10mL THF, and NaH (0.069g, 2.88mmol) was slow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com