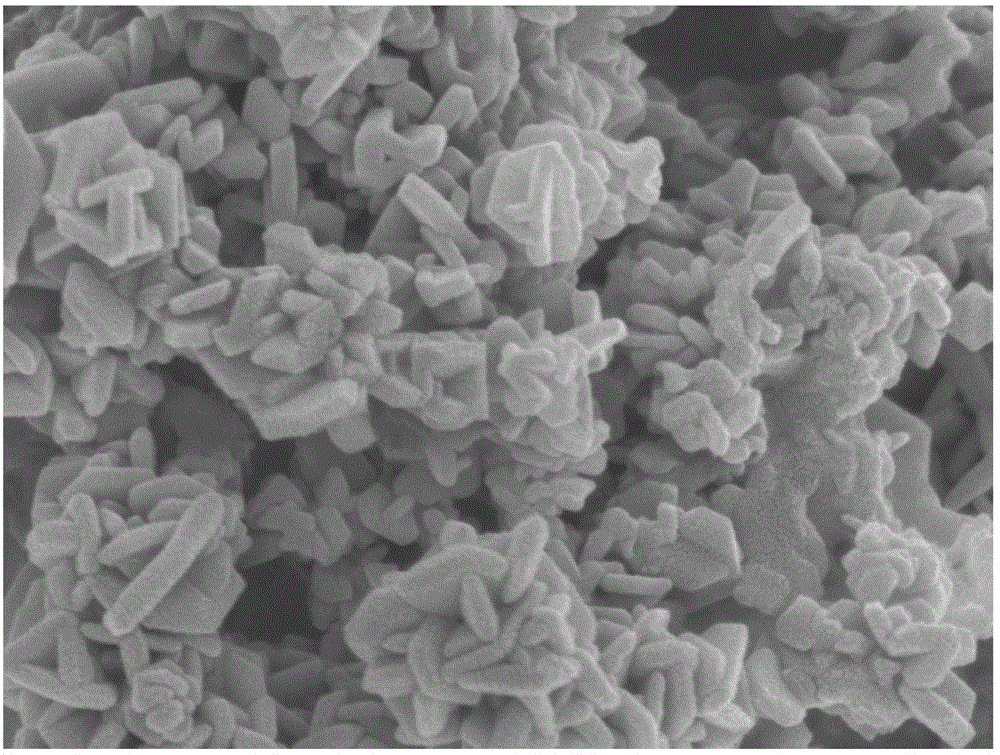
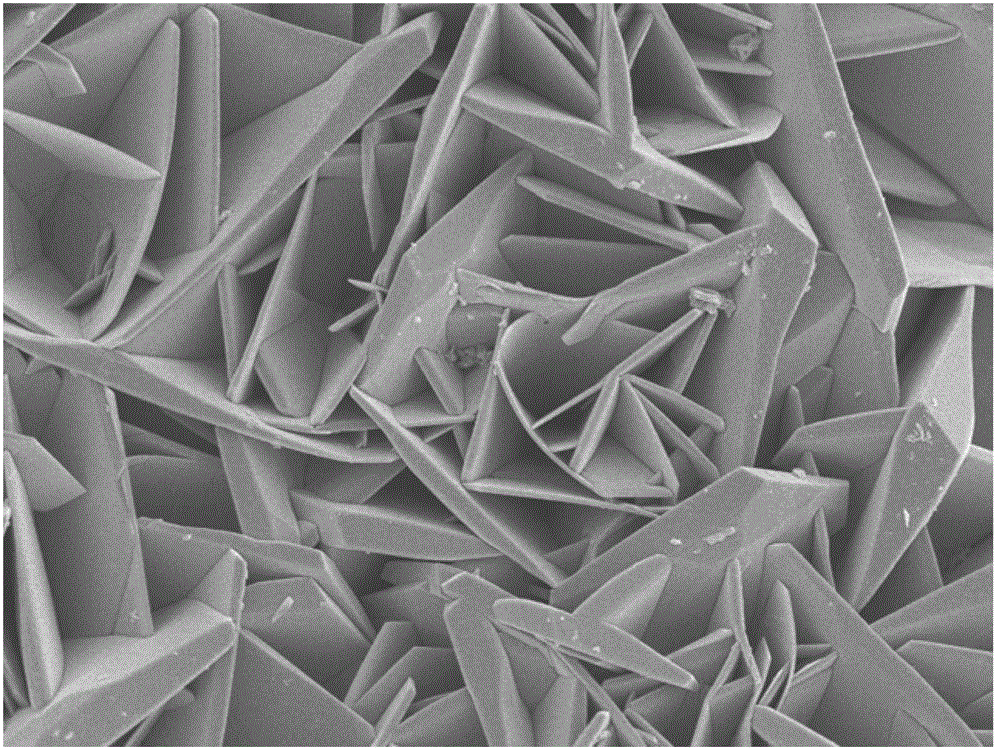
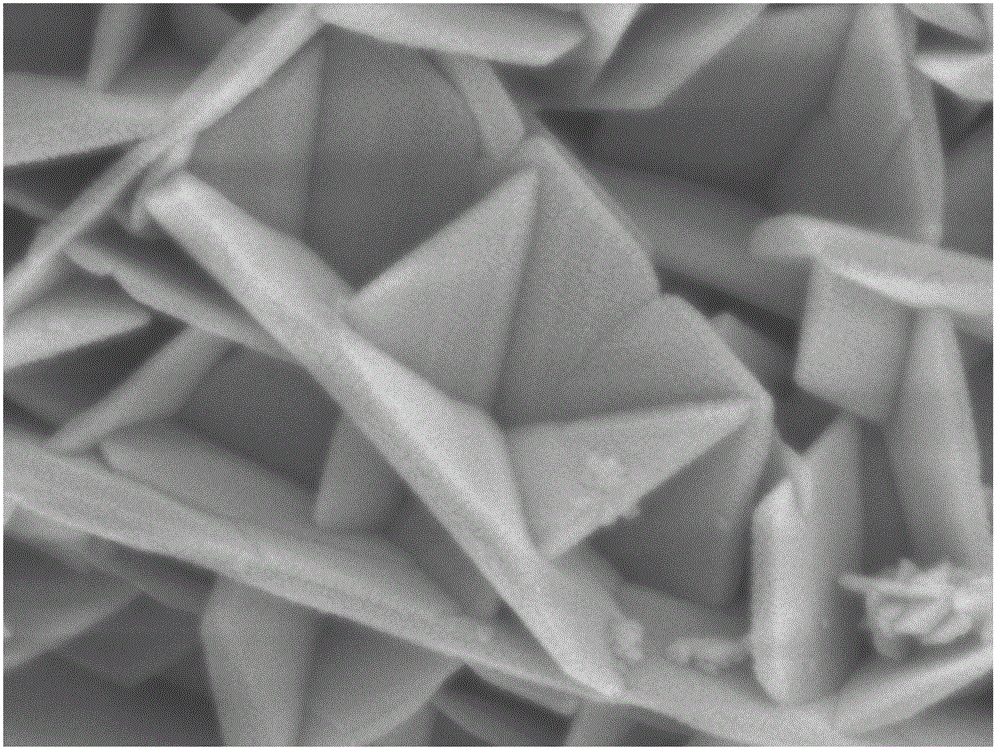
Method for preparing lithium cobalt oxide nanosheets
A nanosheet, lithium cobalt oxide technology, applied in nanotechnology, chemical instruments and methods, cobalt compounds, etc., can solve the problems of lithium ion insertion and migration difficulties, affecting electrical properties, and large particle size of lithium cobalt oxide.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1) Take Co (Ac) 2 4H 2 O 1.4g and 16mL of 5M NaOH solution were mixed, placed in a 20mL hydrothermal kettle, sealed and placed in an oven at 170°C for 5 hours. After taking out the hydrothermal kettle and cooling naturally in the air to room temperature, a dark blue cobalt-containing solution was obtained.
[0015] 2) After taking out the dark blue solution, centrifuge to remove the black precipitate in the lower layer, then add 0.1g LiCl, add the mixed solution into a 20mL hydrothermal kettle, seal the hydrothermal kettle tightly and place it in an oven at 150°C for 10h. After taking out the hydrothermal kettle, it was naturally cooled to room temperature in the air, and the black precipitate was repeatedly washed with deionized water and absolute ethanol, and dried at room temperature to obtain flaky LiCoO 2 nanomaterials.
[0016] 3) Assemble the obtained lithium cobalt oxide into a 2025-type button battery, and charge and discharge at 5mA.h.g -1 The rate is test...
Embodiment 2
[0018] 1) Take CoSO 4 .7H 2 O 1.4g and 15mL of 10M NaOH solution were mixed, placed in a 20mL hydrothermal kettle, sealed and placed in an oven at 190°C for 8 hours. After taking out the hydrothermal kettle and cooling naturally in the air to room temperature, a dark blue cobalt-containing solution was obtained.
[0019] 2) After taking out the dark blue solution, centrifuge to remove the black precipitate in the lower layer, then add 0.2g LiCl, put the mixed solution into a 20mL hydrothermal kettle, seal the hydrothermal kettle tightly and place it in an oven at 180°C for 8 hours. After taking out the hydrothermal kettle, it was naturally cooled to room temperature in the air, and the black precipitate was repeatedly washed with deionized water and absolute ethanol, and dried at room temperature to obtain flaky LiCoO 2 nanomaterials.
[0020] 3) Assemble the obtained lithium cobalt oxide into a 2025-type button battery, and charge and discharge at 5mA.g -1 The rate is tes...
Embodiment 3
[0022] 1) Take CoCl 2 .6H 2 O 0.5g and 15mL of 16M NaOH solution were mixed, placed in a 20mL hydrothermal kettle, sealed and heated in an oven at 200°C for 10h. After taking out the hydrothermal kettle and cooling naturally in the air to room temperature, a dark blue cobalt-containing solution was obtained.
[0023] 2) After taking out the dark blue solution, centrifuge to remove the lower black precipitate, then add 0.2g Li 2 SO 4 .H 2 O, add the mixed solution into a 20mL hydrothermal kettle, seal the hydrothermal kettle tightly and place it in an oven at 230°C for 5h. After taking out the hydrothermal kettle, it was naturally cooled to room temperature in the air, and the black precipitate was repeatedly washed with deionized water and absolute ethanol, and dried at room temperature to obtain flaky LiCoO 2 nanomaterials.
[0024] 3) Assemble the obtained lithium cobalt oxide into a 2025-type button battery, and charge and discharge at 5mA.h.g -1 The rate is tested, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com