Compositions of prokaryotic phenylalanine ammonia-lyase variants and methods of using compositions thereof
A technology of algae phenylalanine ammonia lyase and variants, applied to the composition of prokaryotic phenylalanine ammonia lyase variants and the field of using its composition, can solve the lack of structural and biochemical knowledge, and have not yet coordinated issues such as concerted efforts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0258] Also provided herein is a method of purifying prokaryotic PAL or a biologically active fragment, mutant, variant or analog thereof. According to an exemplary first embodiment, the transformed cell mass is grown and disrupted, leaving behind the crude recombinase. Extraneous material is usually separated from the crude block to prevent contamination of the column. Utilize one or more chromatographic resins for chromatographic purification. Next, the purified protein is formulated into a buffer designed to provide stable activity over an extended period of time. In another embodiment, the method of purifying prokaryotic PAL, or a biologically active fragment, mutant, variant or the like thereof, comprises: (a) utilizing a pressure homogenizer (but potentially, by other physical means, such as glass bead lysis) to lyse bacteria containing recombinant prokaryotic PAL or its biologically active fragments, mutants, variants, or the like; (b) heat treatment; (c) using a seco...
Embodiment 1
[0262] Cloning of Nostoc punctatus and Anabaena variabilis PAL
[0263] DNA manipulation
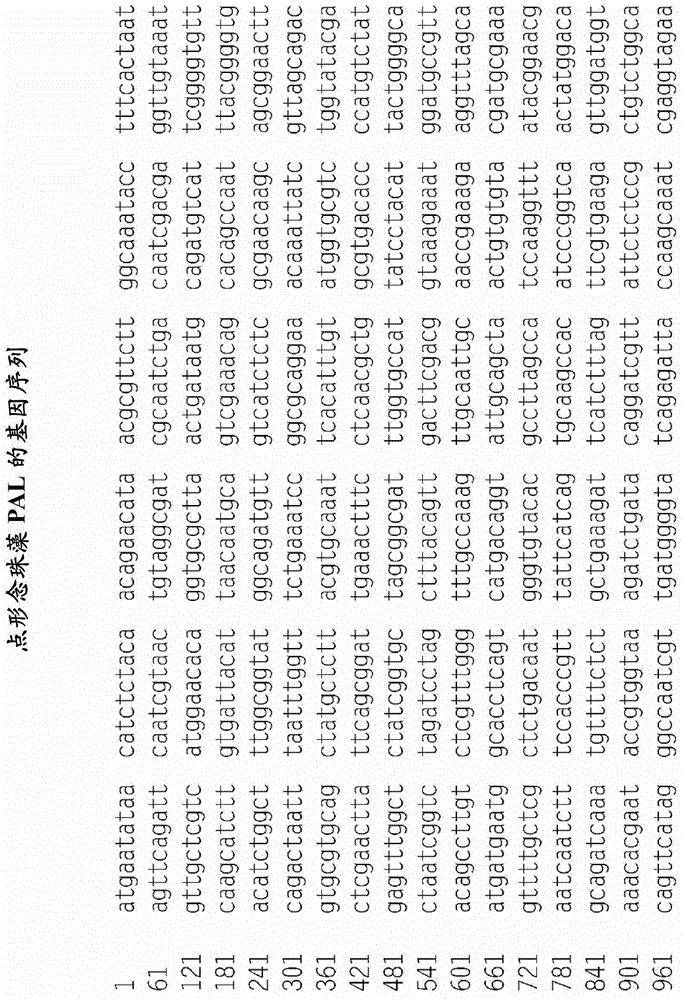
[0264] Genomic DNA of Nostoc punctatus was purchased from ATCC (29133D) and the PAL gene (ZP_00105927) was amplified by PCR with primers 5'-CACTGTCATATGAATAAACATCTCTACAACAGAACAT-3' (SEQ ID NO: 12) and 5'-GACAGTGGCGGCCGCTCACGTTGACTTTAAGCTCGAAAAAATATG-3' (SEQ ID NO: 13) increase. The resulting PCR product was digested with NdeI and NotI and the 1.7kb fragment was ligated into pET-28a(+) and pET-30a(+) (Novagen) with and without N-His tagging, respectively .
[0265] Anabaena variabilis cells were purchased from ATCC (29413). Genomic DNA was extracted (Qiagen) and the PAL gene (YP_324488) was amplified by SOE-PCR to remove the NheI site. Primer 1 (5'-CACTGTGCTAGCATGAAGACACTATTCTCAAGCACAAAG-3') (SEQ ID NO: 14) and primer 2 (5'-GGAAATTTCCTCCATGATAGCTGGCTTGGTTATCAACATCAATTAGTGG-3') (SEQ ID NO: 15) were used to amplify nucleotides 1-1190 and primer Primer 3 (5'-CCACTAATTGATGTTGATAACCAAGCCA...
Embodiment 2
[0272] Purification of NpPAL and AvPAL
[0273]The culture was centrifuged at 5,000 g for 20 min in a tabletop centrifuge and the supernatant was discarded. Cell pellets are usually frozen at –70°C prior to further processing. After thawing, the pelleted cells were suspended to approximately 80 optical density units (600 nm) in TBS (25 mM Tris, 150 mM NaCl, pH 7.8). Cells were lysed by passing through the APV pressure homogenizer twice at 12-14,000 psi. The crude lysate was then heat treated at 55 °C for 2 h. The lysate was centrifuged at 10,000 g for 30 min, and the supernatant was retained and filtered with a 0.2 μm vacuum filter (Corning).
[0274] PAL was purified from the clarified lysate by passing it sequentially through a Butyl 650M column (Tosoh BioSciences) followed by a MacroPrep High Q column (BioRad). The products eluted by both methods of SDS PAGE and reverse phase HPLC showed high purity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com