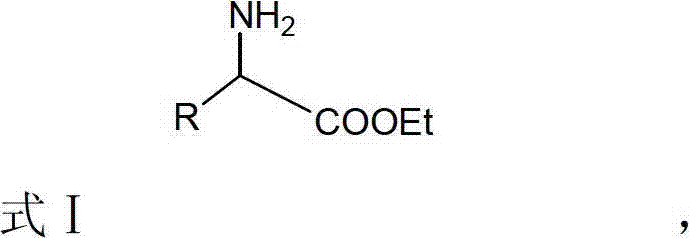
Fenofibric urethan, preparation method and application thereof
A technology of amino acid ethyl ester salt and fenofibric acid, which is applied in the field of medicine, can solve the problems of affecting drug efficacy and poor water solubility, and achieve the effects of small side effects, high bioavailability, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
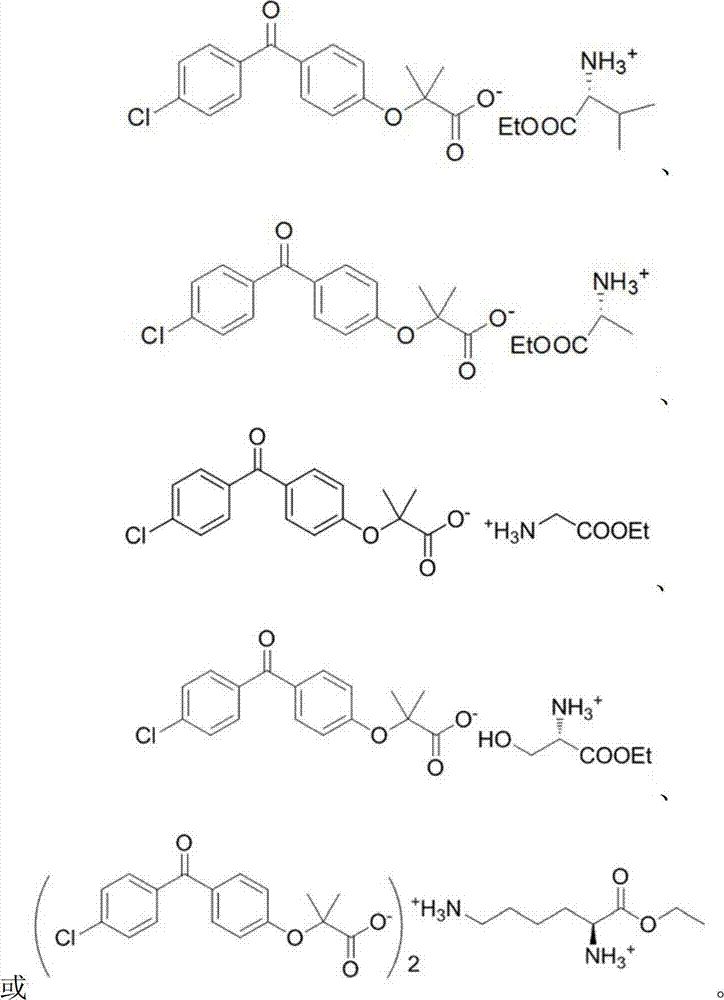
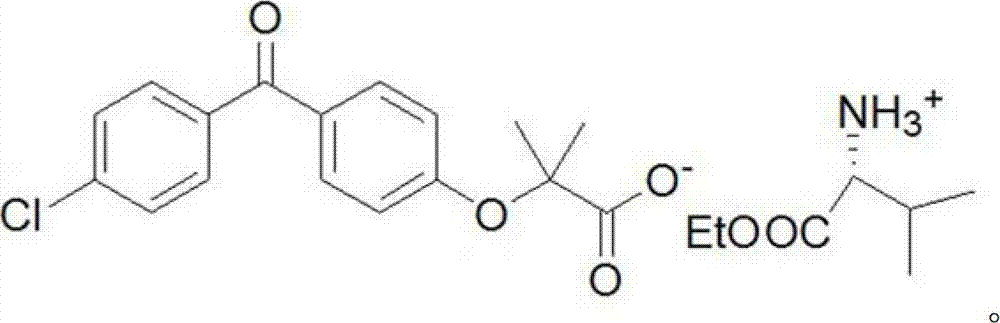
[0021] The fenofibric acid amino acid ethyl ester salt provided in this embodiment is:
[0022]
[0023] The preparation method is as follows: add 0.8g fenofibrate acid (2.5mmol) into 20ml ethyl acetate, stir at room temperature, add 5ml ethyl acetate solution containing 0.38g L-valine ethyl ester (2.6mmol), Wash with 3ml of ethyl acetate and add to the reaction system, the reaction system gradually becomes clear, and then a white precipitate gradually precipitates, the reaction system is heated to 50°C and kept for 20min, adding diethyl ether dropwise to make the reaction system clear, and slowly lowered to room temperature, about It takes 1.5 hours, and it is stirred at room temperature for 1 hour, filtered, the filter cake is washed with ether, and dried to obtain 0.35 g of a white solid, with a yield of 29%. The solubility of this compound in 1ml of pure water is about 3mg, which is slightly soluble. The nuclear magnetic analysis data of this compound are as follows: ...
Embodiment 2
[0025] The fenofibric acid amino acid ethyl ester salt provided in this embodiment is:
[0026]
[0027] Its preparation method is: add 0.9g fenofibric acid (2.8mmol) into 20ml ethyl acetate, under stirring at room temperature, add 5ml of ethyl acetate solution containing 0.34g L-alanine ethyl ester (2.9mmol), Wash with 3ml of ethyl acetate and add to the reaction system, the reaction system gradually becomes clear, and then a white precipitate gradually precipitates, the reaction system is heated to 50°C and kept for 20min, adding diethyl ether dropwise to make the reaction system clear, and slowly lowered to room temperature, about 1.5 hours, and stirred at room temperature for 1 hour, filtered, the filter cake was washed with ether, and dried to obtain 0.50 g of white solid, yield 40%. The solubility of this compound in 1ml of pure water is greater than 100mg, which is easily soluble. The nuclear magnetic analysis data of this compound are as follows: 1 H-NMR (D 2 O):...
Embodiment 3
[0029] The fenofibric acid amino acid ethyl ester salt provided in this embodiment is:
[0030]
[0031] Its preparation method is: add 0.9g fenofibric acid (2.8mmol) into 20ml ethyl acetate, under normal temperature stirring, add 5ml ethyl acetate solution containing 0.30g glycine ethyl ester (2.8mmol), and add 3ml ethyl acetate After washing the ester, add the reaction system, the reaction system gradually becomes clear, and then gradually precipitates a white precipitate, the reaction system is heated to 50 degrees and kept for 20 minutes, and diethyl ether is added dropwise to make the reaction system clear, slowly lowered to room temperature, about 1.5 hours, and Stir at room temperature for 1 hour, filter, wash the filter cake with ether, and dry to obtain 0.52 g of white solid, yield 43%. The solubility of this compound in 1ml of pure water is greater than 100mg, which is easily soluble. The nuclear magnetic analysis data of this compound are as follows: 1 H-NMR (D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com