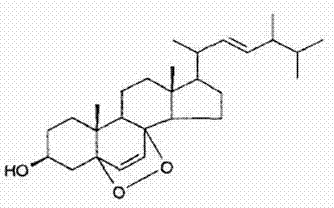
Application of 5alpha-8alpha-ergosterol peroxide-6,22(E)-diene-3beta-alcohol to preparation of anti-tumor drug
An anti-tumor drug, the technology of peroxygen, which is applied in the field of medicine, can solve the problem that the killing effect of tumor stem cells has never been discovered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Isolation and purification of 5α-8α-peroxyergosta-6,22(E)-dien-3β-ol from Ganoderma lucidum spores
[0024] 1. Collect 10 kg of Ganoderma lucidum spores, break the wall by enzymatic method, and then extract by carbon dioxide supercritical extraction apparatus. The extraction conditions are: extraction pressure 25MPa±1MPa, temperature 40oC±5oC; carbon dioxide flow rate 175L / h±15L / h; separation The pressure is 6Mpa±1Mpa, the temperature of the secondary separation tank is 40 oC±2 oC, the temperature of the third separation tank is 45 oC±2 oC, and the extraction time is 4 hours; The extract, namely Ganoderma lucidum spore oil, is 1300g in total, and stored below 16°C for later use.
[0025] 2. Take 210g of Ganoderma lucidum spore oil, dissolve it with 250ml of petroleum ether (60-90), mix the sample according to the ratio of sample: silica gel = 1:2 (silica gel 160-200 mesh), according to the ratio of sample: silica gel = 1:30 Put on the column (column diameter...
Embodiment 2
[0039] Example 2: Experiment of the inhibitory effect of 5α-8α-peroxyergosta-6,22(E)-dien-3β-ol on human malignant breast tumor cells (MT-1)
[0040] 1. The 5α-8α-peroxyergosta-6,22(E)-diene-3β-alcohol obtained in Example 1 was prepared into a 10mg / ml solution with DMSO, and then diluted to 500ug / ml with DMEM medium ml of sample solution;
[0041] 2. Human malignant breast tumor cells (MT-1) in the logarithmic growth phase were treated with 0.25% trypsin, digested, and re-treated with 10% fetal bovine serum and 1% double antibody (10000U / ml penicillin + 10000U / ml ml streptomycin) suspended in DMEM medium, and then counted to prepare 2×10 5 cells / ml of cell suspension, add 0.96ml of mixed cell suspension to the 12-well plate, and store at 37°C, 5% CO 2 After culturing in the incubator for four hours, add 40ul diluted sample solution containing 5α-8α-peroxyergosta-6,22(E)-diene-3β-ol to each well, and the final concentration is 20ug / ml, set 3 repetitions for each sample solu...
Embodiment 3
[0045] Example 3: Experiment of the inhibitory effect of 5α-8α-peroxyergosta-6,22(E)-dien-3β-ol on human lymphoma cells (Jurkat)
[0046] 1. The 5α-8α-peroxyergosta-6,22(E)-diene-3β-alcohol obtained in Example 1 was prepared into a 10mg / ml solution with DMSO, and then diluted to 500ug / ml with DMEM medium ml of sample solution;
[0047] 2. Human lymphoma cells (Jurkat) in the logarithmic growth phase were treated with 0.25% trypsin, digested, and re-treated with 10% fetal bovine serum and 1% double antibody (10000U / ml penicillin + 10000U / ml streptomycin Suspended in DMEM medium, then counted and prepared into 2×10 5 cells / ml of cell suspension, add 0.96ml of mixed cell suspension to the 12-well plate, and store at 37°C, 5% CO 2 After culturing in the incubator for four hours, add 40ul diluted sample solution containing 5α-8α-peroxyergosta-6,22(E)-diene-3β-ol to each well, and the final concentration is 20ug / ml, set 3 repetitions for each sample solution, set the negative co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com