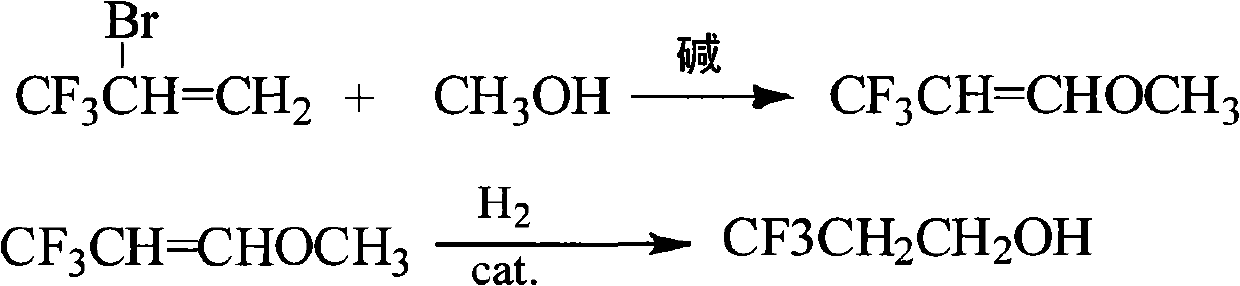
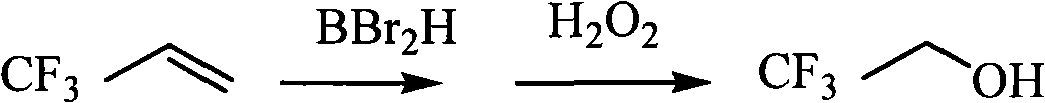Method for synthesizing 3,3,3-trifluoro-propyl alcohol
A technology of trifluoropropanol and a synthesis method, applied in 3 fields, can solve the problems of difficult separation and purification, low reaction yield and the like, and achieve the effects of high reaction yield and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Under stirring, add 96g of methanol and 80g of sodium hydroxide into the reactor, then add 87.5g of 2-bromo-3,3,3-trifluoropropene, heat up to 70°C, react for 6h, cool to room temperature, and material, the reaction solution was washed three times with 100 g of water to neutrality, and methanol, sodium bromide and sodium hydroxide were removed to obtain 44.1 g of 3,3,3-trifluoropropenyl methyl ether with a yield of 70% and a purity of 98%.
[0020] (2) Add 44.1g of 3,3,3-trifluoropropenyl methyl ether, 66.2g of tributyl phosphate and 4.4g of activated Raney Ni catalyst into the autoclave, the pressure of hydrogen filling is 5MPa, under stirring, heat Raise the temperature to 100°C, react for 6 hours, cool to room temperature, remove the reaction solution, filter the reaction solution, separate the catalyst from the reaction solution, distill the obtained filtrate under normal pressure, collect the fraction at 87-89°C to obtain 3,3,3-trifluoropropane Alcohol 35.0g, y...
Embodiment 2
[0034] (1) Under stirring, add 128g of methanol and 80g of sodium hydroxide into the reactor, then add 87.5g of 2-bromo-3,3,3-trifluoropropene, heat up to 70°C, react for 6h, cool to room temperature, and discharge, The reaction liquid was washed three times with 100 g of water to neutrality, and methanol, sodium bromide and sodium hydroxide were removed to obtain 42.8 g of 3,3,3-trifluoropropenyl methyl ether with a yield of 68.0% and a purity of 98%.
[0035] (2) 42.8g 3,3,3-trifluoropropenyl methyl ether, 51.4g dipropyl carbonate and 2.6g activated Raney Ni catalyst are added in the autoclave, the hydrogen pressure is 5MPa, under stirring, the temperature is raised to 100°C, reacted for 6h, cooled to room temperature, removed the reaction liquid, filtered the reaction liquid, separated the catalyst from the reaction liquid, distilled the obtained filtrate under normal pressure, collected 87-89°C fractions, and obtained 3,3,3-trifluoropropanol 32.2 g, yield 81.5%, purity 98%...
Embodiment 3
[0037] (1) Under stirring, add 128g of methanol and 120g of sodium hydroxide into the reactor, then add 87.5g of 2-bromo-3,3,3-trifluoropropene, heat up to 70°C, react for 6h, cool to room temperature, and discharge, The reaction solution was washed three times with 100 g of water to neutrality, and methanol, sodium bromide and sodium hydroxide were removed to obtain 43.5 g of 3,3,3-trifluoropropenyl methyl ether with a yield of 69.1% and a purity of 98%.
[0038] (2) 43.5g 3,3,3-trifluoropropenyl methyl ether, 55.7g diethyl carbonate and 2.2g activated Raney Ni catalyst are added in the autoclave, the pressure of hydrogen filling is 5MPa, under stirring, the temperature is raised to 70°C, reacted for 6h, cooled to room temperature, removed the reaction liquid, filtered the reaction liquid, separated the catalyst from the reaction liquid, distilled the obtained filtrate under atmospheric pressure, collected 87-89°C fractions, and obtained 3,3,3-trifluoropropanol 30.5 g, yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com