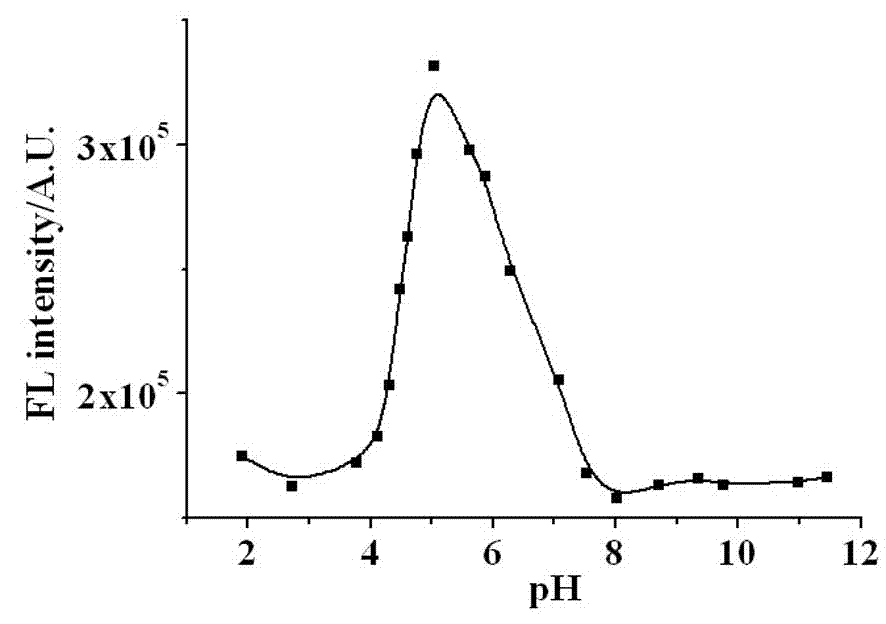
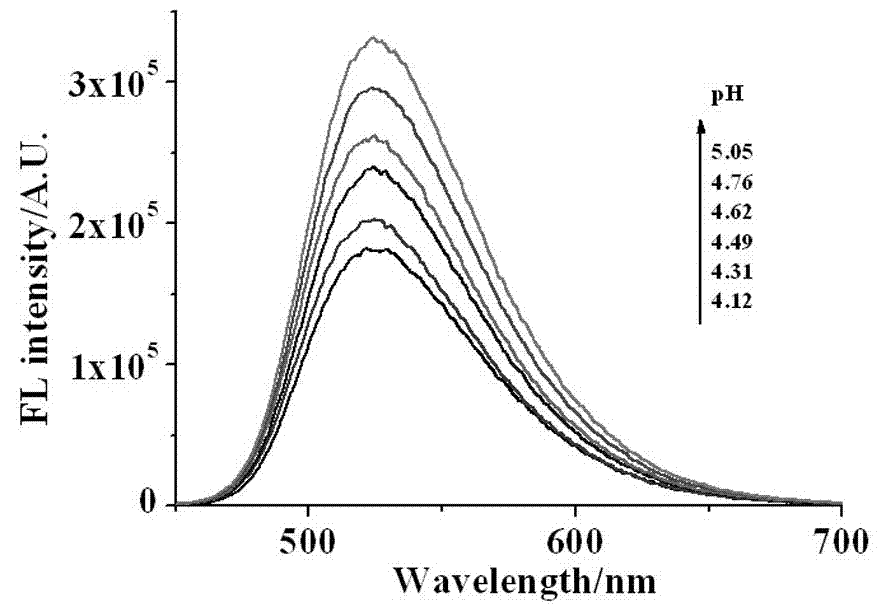
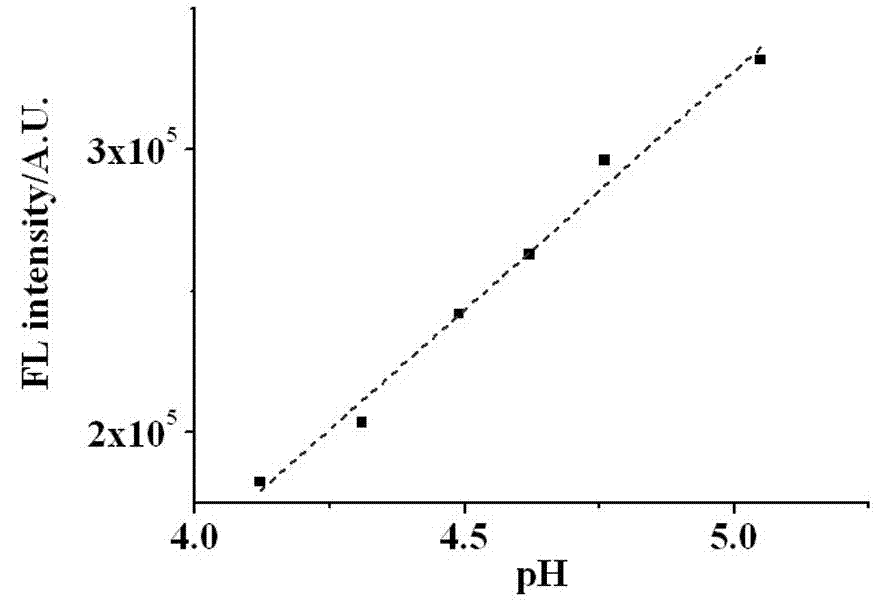
Naphthalimide derivative and application thereof
A technology of naphthalimide and derivatives, which is applied in the application field of naphthalimide derivatives as pH fluorescent molecular switches, can solve problems such as interference, and achieve the effect of short synthesis route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment one: the preparation of intermediate NETB
[0029] With absolute ethanol as solvent, the molar ratio of 4-bromo-1,8-naphthalic anhydride (BNA) to tris(2-aminoethyl)amine (TAEA) n(BNA):n(TAEA)=3.0: 1. First, BNA was dispersed in absolute ethanol in a three-necked flask, stirred, and then TAEA was dropped into it, and mixed evenly. After the addition was completed, the temperature was raised to 60 °C for 4 h. Cool to room temperature, filter, and wash the filter cake with water and absolute ethanol successively to obtain a tan solid, which is dried in vacuo. Separation by silica gel column (mobile phase, V CH3OH :V CHCl3 =1:60) to obtain a pale yellow solid, which was heated to reflux with acetone to wash, filtered while hot to remove the solvent, and a white powder NETB was obtained. Yield 55.5%.
Embodiment 2
[0030] Embodiment two: the preparation of intermediate NETB
[0031] With absolute ethanol as the solvent, the molar ratio of 4-bromo-1,8-naphthalic anhydride (BNA) to tris(2-aminoethyl)amine (TAEA) n(BNA):n(TAEA)=3.1: 1. First, BNA was dispersed in absolute ethanol in a three-necked flask, stirred, and then TAEA was dropped into it, and mixed evenly. After the addition was completed, the temperature was raised to 70 °C for 5 h. Cool to room temperature, filter, and wash the filter cake with water and absolute ethanol successively to obtain a tan solid, which is dried in vacuo. Separation by silica gel column (mobile phase, V CH3OH :V CHCl3 =1:60) to obtain a pale yellow solid, which was heated to reflux with acetone to wash, filtered while hot to remove the solvent, and a white powder NETB was obtained. Yield 59.2%.
Embodiment 3
[0032] Embodiment three: the preparation of intermediate NETB
[0033] With absolute ethanol as the solvent, the molar ratio of 4-bromo-1,8-naphthalic anhydride (BNA) to tris(2-aminoethyl)amine (TAEA) n(BNA):n(TAEA)=3.1: 1. First, BNA was dispersed in absolute ethanol in a three-necked flask, stirred, and then TAEA was dropped into it, and mixed evenly. After the addition was completed, the temperature was raised to 78 °C for 5 h. Cool to room temperature, filter, and wash the filter cake with water and absolute ethanol successively to obtain a tan solid, which is dried in vacuo. Separation by silica gel column (mobile phase, V CH3OH :V CHCl3 =1:60) to obtain a pale yellow solid, which was heated to reflux with acetone to wash, filtered while hot to remove the solvent, and a white powder NETB was obtained. Yield 56.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com