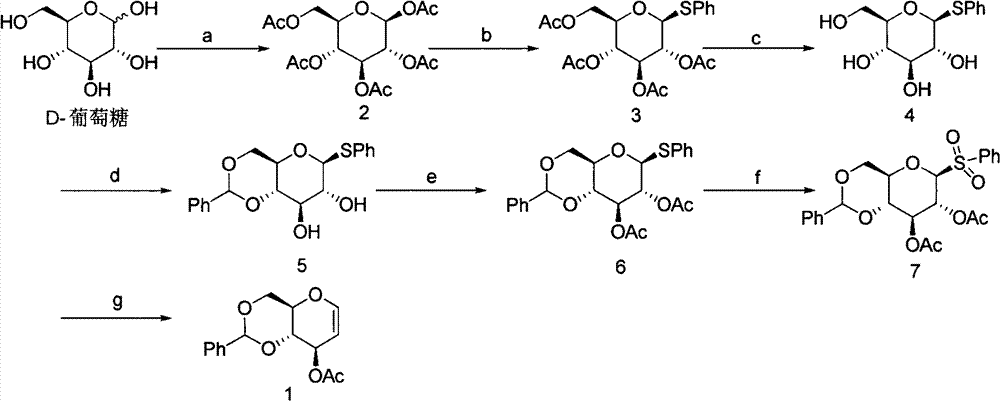
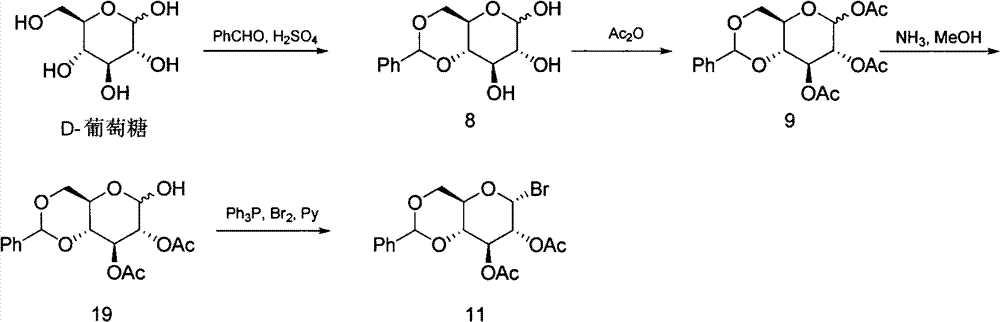
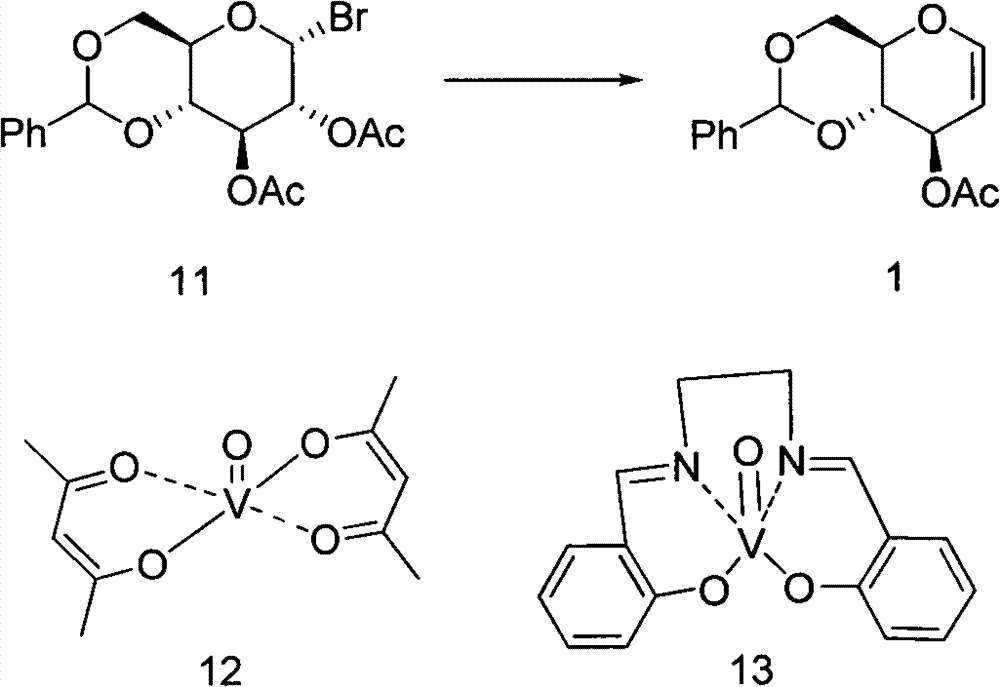
Preparation method of 4,6-O-benzylene-3-O-acetyl-D-glucal
A technology of glucone and acetyl group, applied in 4 fields, can solve problems such as high cost of personal safety reagents, unfavorable large-scale production, unfavorable environmental protection, etc., and achieves the effects of easy operation, little pollution, and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 2.5 g (6 mmol) of 4,6-O-benzylidene-3-O-acetyl-1-bromo-D-glucose (compound 11) and dissolve it in 7.5 mL of dichloromethane. Add Zn (1.95g, 30mmol), NH 4 Cl(1.6g, 30mmol), VO(AcAc) 2 (3mg, 0.012mmol) and AcOH (0.03mL, 0.6mmol), stirred at 10°C for 24h. TLC monitoring, the reaction is complete. Filter, wash the filter residue with 10mL dichloromethane, dilute the filtrate with 20mL dichloromethane, then wash twice with 15mL water, once with 15mL of saturated sodium bicarbonate solution, once with 15mL of saturated saline, once with anhydrous Na 2 SO 4 dry. After filtration, the filtrate was spin-dried and purified by silica gel column chromatography to obtain 0.38 g of a white solid (compound 1) with a yield of 57%. The purity was greater than 99% as determined by HPLC.
[0023] Its structural identification data are as follows:
[0024] 1 H NMR: δ6.39 (dd, 1H, J 1,2 =6.1,J 1,3 =1.7Hz, H-1), 4.80(dd, 1H, H-2), 5.45(dt, 1H, J 2,3 =1.9,J 3,4 =8.8Hz, H-3), ...
Embodiment 2
[0027] Weigh 1.0 g (2.4 mmol) of 4,6-O-benzylidene-3-O-acetyl-1-bromo-D-glucose (compound 11) and dissolve it in 15 mL of MeCN. Add Zn (1.56g, 24.08mmol), NH 4 Cl (1.29g, 24.08mmol), VO(AcAc) 2 (6.5mg, 0.024mmol) and AcOH (0.13mL, 2.4mmol), stirred at 30°C for 12h. TLC monitoring, the reaction is complete. Filter, wash the filter residue with 10mL ethyl acetate, dilute the filtrate with 25mL ethyl acetate, then wash twice with 15mL water, once with 15mL of saturated sodium bicarbonate solution, once with 15mL of saturated saline, once with anhydrous Na 2 SO 4 dry. After filtration, the filtrate was spin-dried and purified by silica gel column chromatography to obtain 0.57 g of a white solid (compound 1) with a yield of 85.6%. The purity was greater than 99% as determined by HPLC.
Embodiment 3
[0029] Weigh 1.0 g (2.4 mmol) of 4,6-O-benzylidene-3-O-acetyl-1-bromo-D-glucose (compound 11) and dissolve it in 10 mL of MeCN. Add Zn (1.56g, 24.08mmol), NH 4 Cl (1.29g, 24.08mmol), VO(Salon) (15.8mg, 0.048mmol), AcOH (1.36mL, 24mmol), stirred at 25°C for 10h. TLC monitoring, the reaction is complete. Filter and wash the filter residue with 10 mL of ethyl acetate. After diluting the filtrate with 20 mL of ethyl acetate, wash it twice with 15 mL of water, once with 15 mL of saturated sodium bicarbonate solution, once with 15 mL of saturated saline, and once with anhydrous Na 2 SO4 dry. After filtration, the filtrate was spin-dried and purified by silica gel column chromatography to obtain 0.59 g of a white solid (compound 1) with a yield of 89.5%. The purity was greater than 99% as determined by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com