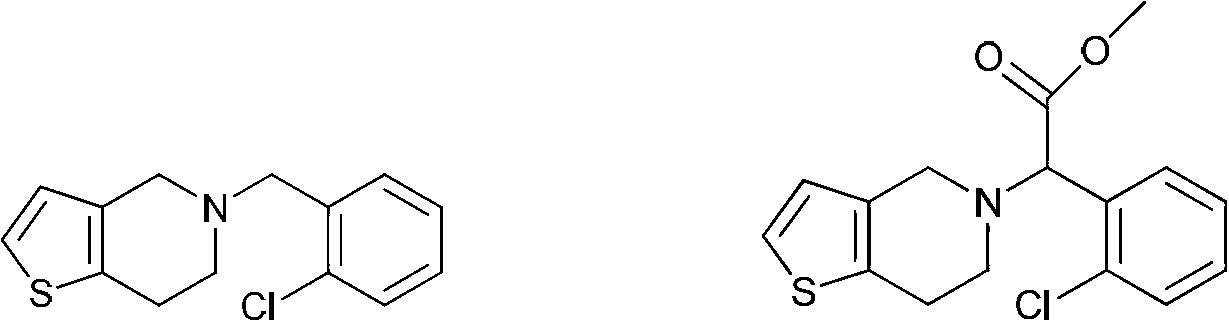
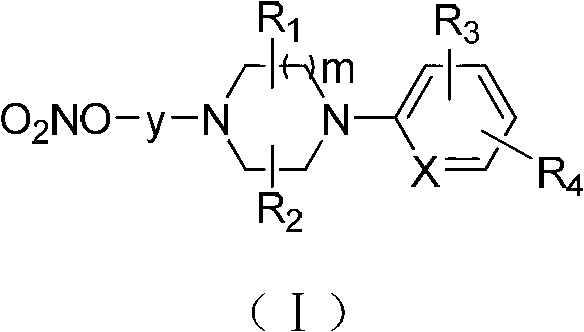
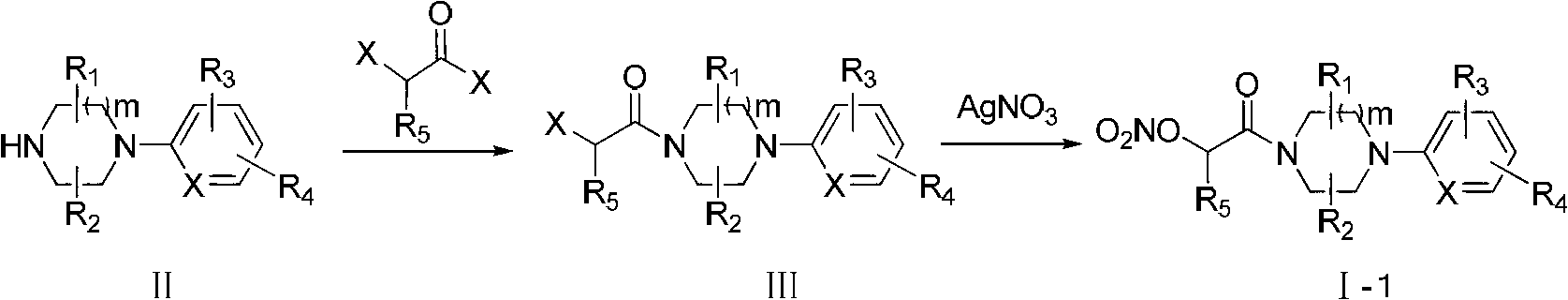
1,4-disubstituted piperazine derivatives and their preparation method and use
A technology of piperazine and compounds, applied in the field of anti-platelet drugs, compounds with anti-platelet aggregation and their preparation, can solve the problems of clopidogrel resistance and achieve the effect of inhibiting platelet aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 2-(4-(3-Chloropyridin-2-yl)piperazin-1-yl)-2-oxoethyl nitrate (compound Ⅰ-1-1)
[0085]
[0086] Add intermediate III-1 (2.4g, 0.01mol) to the reaction flask equipped with stirring, condenser and thermometer, dissolve in anhydrous acetonitrile (20m1), and add anhydrous acetonitrile solution of silver nitrate (2.0g, 0.012mol) (10m1), refluxed for 5h under stirring in the dark, TLC showed that the reaction was complete and cooled to room temperature, and the solvent was evaporated to dryness under reduced pressure. Dichloromethane (20ml) was added to the residue, stirred for 10min, filtered, and the filtrate was evaporated to dryness under reduced pressure. Add absolute ethanol (30ml), evaporate to dryness under reduced pressure after activated carbon decolorization, and dry under reduced pressure at room temperature overnight to obtain light yellow transparent oil Ⅰ-1-1 (2.6g, yield 90%), purity 98.5% (HPLC method ). HRMS(m / z)[M+H] + : 302.0596.
[0087] Referring...
Embodiment 2
[0090] 2-(4-(3-Chloropyridin-2-yl)piperazin-1-yl)ethyl nitrate (compound Ⅰ-2-1)
[0091]
[0092] Mix 6.5ml of fuming nitric acid and 19.5ml of acetic anhydride in a reaction flask equipped with stirring, condenser and thermometer, and control the temperature at -15°C, and dropwise add intermediate IV-1 (2.9g, 0.01mol) Tetrahydrofuran (100ml) solution was added dropwise, then slowly raised to room temperature, reacted for 4h, and ice water was added dropwise to stop the reaction. The reaction mixture was dissolved in 200ml of ethyl acetate, washed successively with 200ml of water, 200ml of saturated sodium bicarbonate solution, and then washed with 200ml of water and 200ml of saturated sodium chloride solution. The organic layer was dried over anhydrous magnesium sulfate. Concentrated under reduced pressure, the crude product was purified by column chromatography (v (petroleum ether): v (ethyl acetate) = 1:2] to obtain 1.4 g of yellow oil with a yield of 50% and a purity o...
Embodiment 3
[0096] Compound Ⅰ-1-1 into hydrochloride: Take 3.2 g of the light yellow oily substance of Ⅰ-1-1 and dissolve it in 15 mL of absolute ethanol. Cool in an ice-water bath to 5°C, add 11.1% ethanol hydrochloric acid solution dropwise until the pH is 2, and continue stirring for about 1 h in an ice-water bath. Filter to obtain a light yellow solid, which is dried in vacuo.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com