siRNA (small interfering ribose nucleic acid) micro emulsion carrier and preparation method of siRNA micro emulsion carrier
A technology of microemulsion and carrier, which is applied in the field of medicine to achieve the effects of prolonging residence time, improving stability and protecting stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
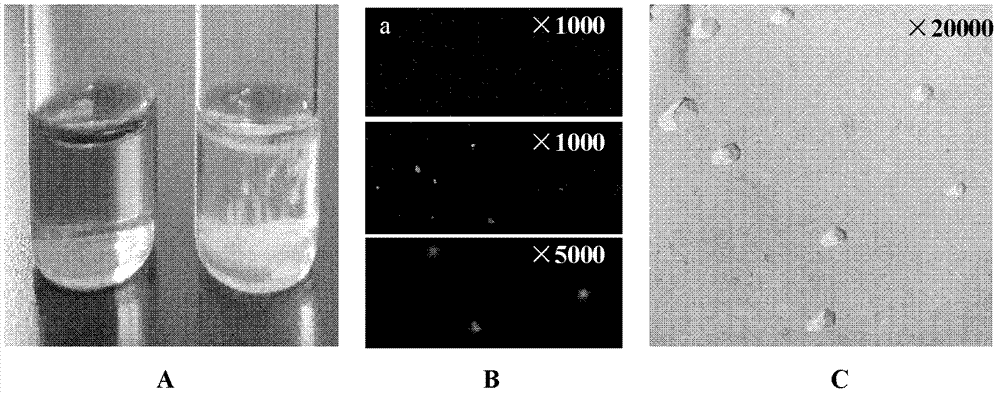
[0038] Embodiment 1: Preparation of NC-FAM siRNA microemulsion
[0039] In order to prevent RNase contamination, all medicines, containers, and materials used in the microemulsion preparation process must be treated without enzymes or must be enzyme-free products. The oil phase and two emulsifiers used in the preparation of microemulsions were baked in an oven at 75°C for 8 hours; metal containers were baked in an oven at 180°C for 3 hours; plastics, rubber and other materials that are not resistant to high temperatures were soaked in 0.1% DEPC water 24h, can withstand high temperature and high pressure and then 120 ℃, 20min sterilization; disposable materials use enzyme-free products.
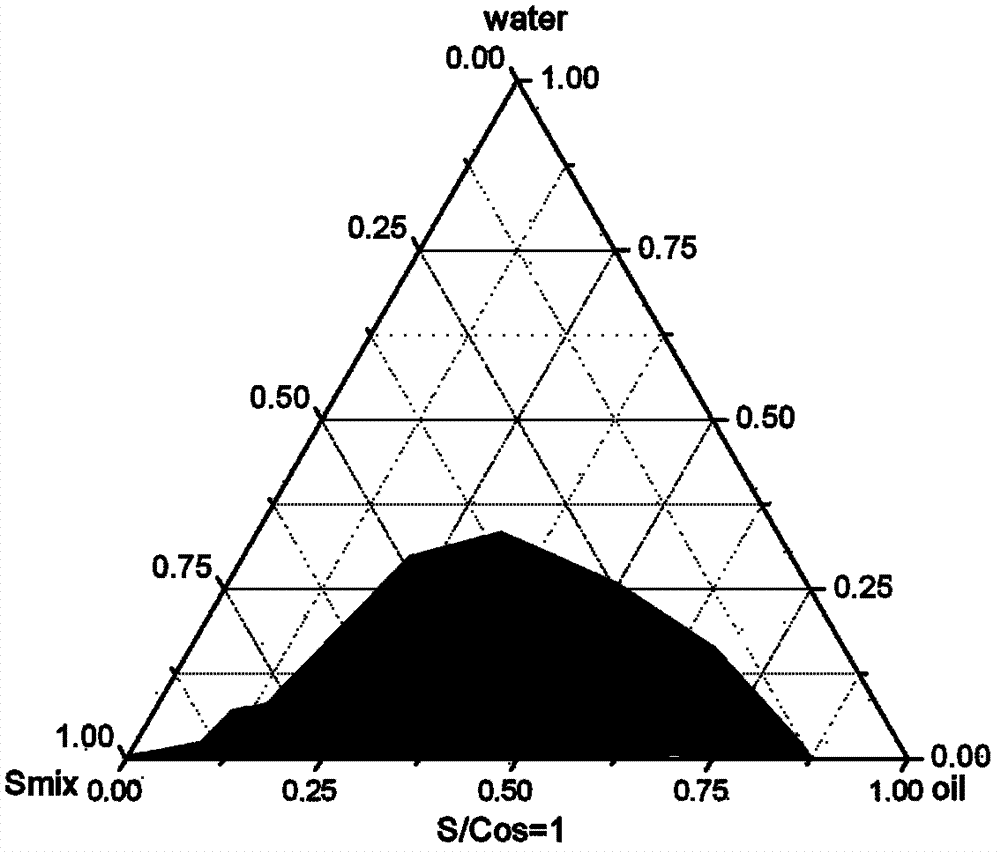
[0040] NC-FAM siRNA microemulsion formulation and ratio:
[0041]
[0042] The preparation method is as follows: design siRNA (NC siRNA) of any sequence, modify it with carboxyfluorescein (FAM) to form NC-FAM siRNA, and dissolve it in enzyme-free water. Weigh the oil, emulsifier and co-em...
Embodiment 2
[0044] Embodiment 2: Preparation of eotaxin siRNA microemulsion
[0045] In order to prevent RNase contamination, all medicines, containers, and materials used in the microemulsion preparation process must be treated without enzymes or must be enzyme-free products. The oil phase and two emulsifiers used in the preparation of microemulsions were baked in an oven at 75°C for 8 hours; metal containers were baked in an oven at 180°C for 3 hours; plastics, rubber and other materials that are not resistant to high temperatures were soaked in 0.1% DEPC water 24h, can withstand high temperature and high pressure and then 120 ℃, 20min sterilization; disposable materials use enzyme-free products.
[0046] Formula and ratio of Eotaxin siRNA microemulsion:
[0047]
[0048]
[0049] The preparation method is: design eotaxin-specific siRNA, i.e. eotaxin siRNA (design according to the Mus musculus small chemokine (C-C motif) ligand 11 (CCL11) mRNA (982bp) whose sequence Accession Num...
Embodiment 3
[0050] Embodiment 3: Preparation of STAT6siRNA microemulsion
[0051] SATA6siRNA microemulsion formula and ratio:
[0052]
[0053]STAT6 (Signal transducers and activators of transcription 6, signal transduction and transcription activator 6)-specific siRNA, that is, STAT6siRNA, the sequence refers to the report of Rippmann JF et al. (Rippmann JF, Schnapp A, Weith A, Hobbie S. Gene silencing with STAT6specific siRNAs blocks eotaxin release in IL-4 / TNFalpha stimulated human epithelial cells. FEBS Lett.2005Jan 3; 579(1): 173-8.), Sense strand: 5'CAG UUC CGC CAC UUG CCA ATT 3'; Antisense strand: 5'UUG GCA AGU GGC GGA ACU GTT3'. All the other are with embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com