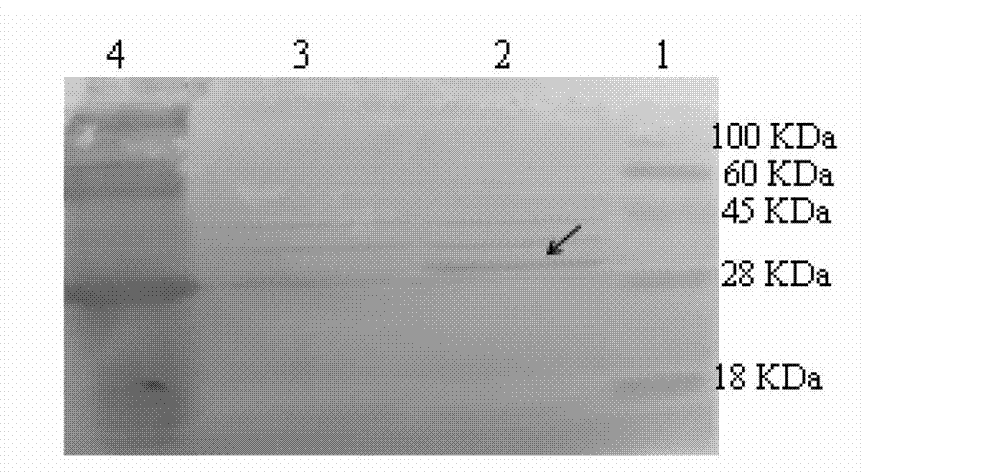
Method for expressing eimeria acevulina 3-1E protein in lactococcus lactis
A technology of Lactococcus lactis and Eimeria, applied in the field of expressing Eimeria gallinarum 3-1E protein, can solve the problem of inability to express Eimeria gallinarum sporozoite/merozoite stage surface antigen 3 -1E and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
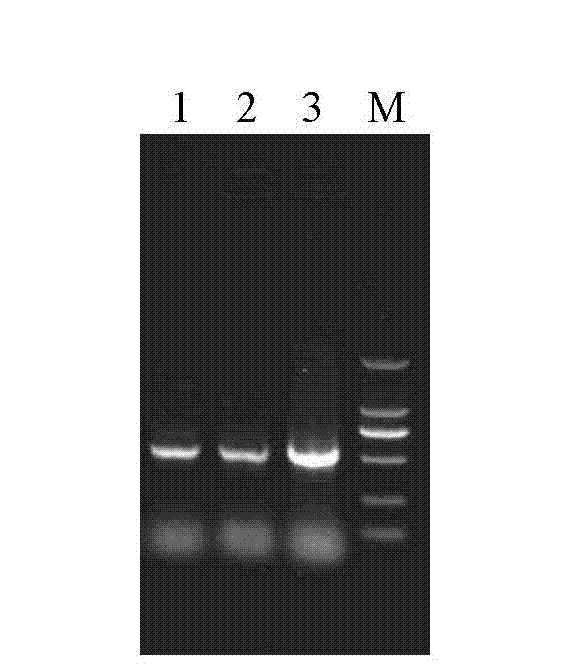
[0015] Specific embodiment one: In this embodiment, the method for expressing the Eimeria gallinacea 3-1E protein in Lactococcus lactis is carried out according to the following steps:
[0016] 1. Optimize the 3-1E gene sequence of Eimeria gallissima according to the codon preference of Lactococcus lactis, obtain the artificially synthesized 3-1E gene, and introduce BamH at its 5' end and 3' end respectively 2 and the recognition sequence of Xba 2, and then connected with the cloning vector pUC57 and transformed into Escherichia coli DH5α competent cells, and screened the positive transformant pUC57-3-1E;
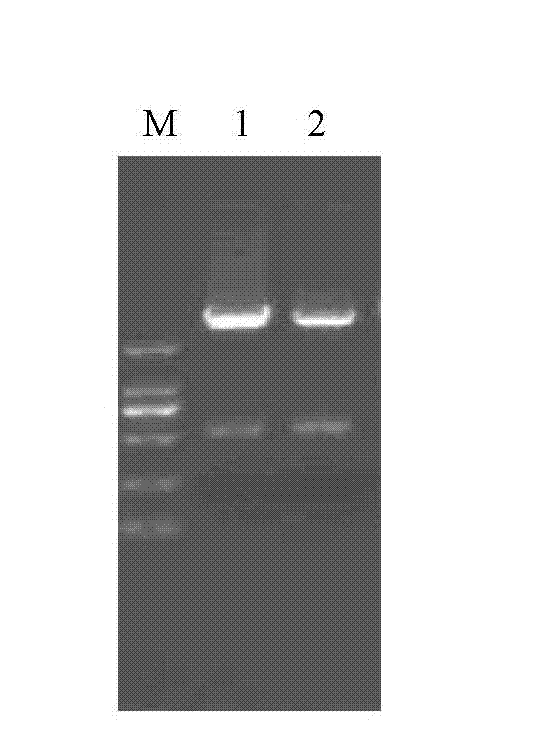
[0017] 2. Digest pUC57-3-1E and Escherichia coli-lactic acid bacteria shuttle expression vector pTX8048 with BamH 3 and Xba3 respectively, recover the 3-1E fragment and vector fragment respectively, and construct the recombinant expression vector pTX8048 after ligation with T4 DNA ligase -3-1E;
[0018] 3. Transform pTX8048-3-1E into the competent cells of Lactococcus lact...
specific Embodiment approach 2
[0023] Specific embodiment 2: This embodiment is a further step of double enzyme digestion of pUC57-3-1E in step 2 of the method for expressing Eimeria gallinacea 3-1E protein in Lactococcus lactis described in specific embodiment 1 Instructions, the pUC57-3-1E double digestion system in step 2 is as follows:
[0024] Element
[0025] The enzyme digestion reaction condition was 37°C for 3h. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0026] Specific embodiment three: this embodiment is a further description of the expression vector pTX8048 double enzyme digestion in step two of the method for expressing Eimeria gallinacea 3-1E protein in Lactococcus lactis described in specific embodiment one , the system of double digestion of the expression vector pTX8048 in step 2 is as follows:
[0027] Element
[0028] The enzyme digestion reaction condition was 37°C for 3h. Others are the same as in the first embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com