Preparation of novel green chelating agent glutamic diacetate tetracetic acid metal salt
A technology of tetrasodium glutamic acid and tetrasodium acetate, which is applied in the field of synthesis of tetrasodium glutamic acid diacetate, can solve the problems of high waste water treatment cost, poor product color, easy-to-corrosion equipment, etc., and achieve low cost and non-sticky products , easy-to-handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
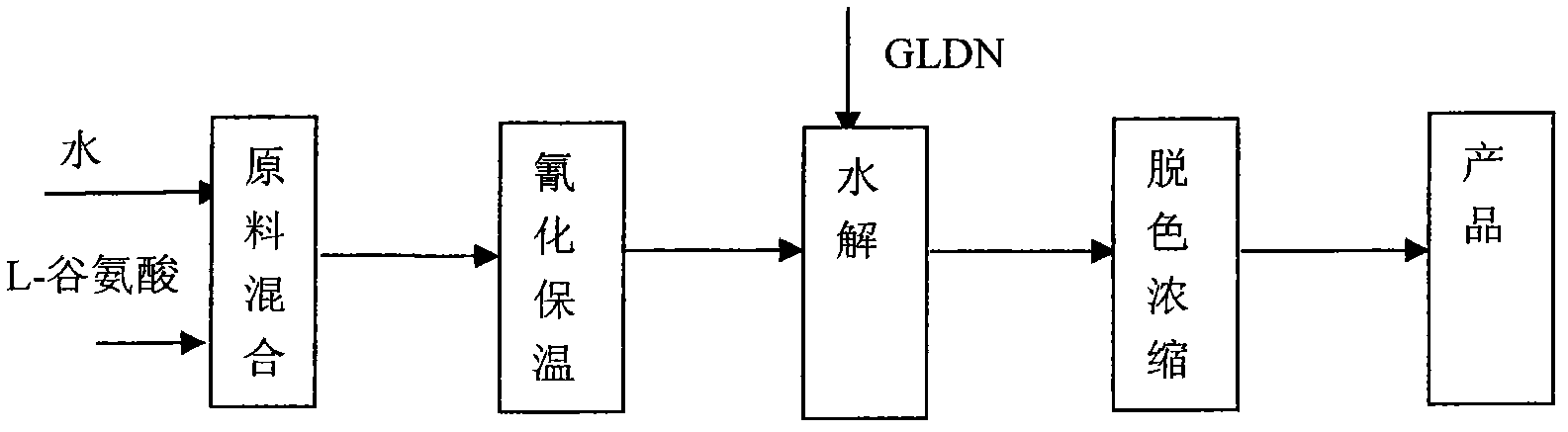
[0028] Add 100g of L-glutamic acid and 200g of water into the four-neck flask, stir to dissolve, then cool down to 30°C, add 102.7g of 2-chloroacetonitrile and 105.8 of pyridine within 0.5-2h; after the addition, stir at this temperature for 3h , obtain the aqueous solution of GLDN; Detect purity with HPLC and can reach more than 95% (mobile phase: methanol: water=15: 85); Add GLDN solution in the four-necked bottle, add 10g zinc chloride, reflux reaction 3h, then add 356g of 30% NaOH, to produce GLDA-4Na, after the addition, it is necessary to reflux and discharge ammonia for 2 hours; the obtained GLDA-4Na solution has a certain color, add activated carbon for decolorization for 3 hours, and concentrate to obtain 515g of GLDA-4Na with a concentration of 41% after removing the activated carbon. The rate is 90.02%.
Embodiment 2
[0030] Add 100g of L-glutamic acid and 300g of water into the four-necked flask, stir to dissolve, then cool down to 50°C, add 150.4g of 2-chloroacetonitrile and 195.7g of triethylamine to it in about 1.5h; after stirring for 5h, the product of GLDN is obtained. Aqueous solution; Detect purity with HPLC and can reach more than 96% (mobile phase: methanol: water=15: 85); The following hydrolysis, decolouring, concentration steps are the same as the second half of example 1; Finally obtaining 520g concentration is 40.58% GLDA- 4Na, yield 89.96%.
Embodiment 3
[0032] The part to obtain the GLDN solution is the same as the previous part of Example 2; add the GLDN solution to the four-necked bottle, add 50g of zinc chloride, and reflux for 3 hours, then add 434g of 30% NaOH to generate GLDA-4Na, and reflux and exhaust ammonia for 6 hours after the addition ; The obtained GLDA-4Na solution has a certain color, adding activated carbon for decolorization for 0.5h, and concentrating after removing the activated carbon to obtain 502g of GLDA-4Na with a concentration of 46.12%, and the yield is 98.70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com