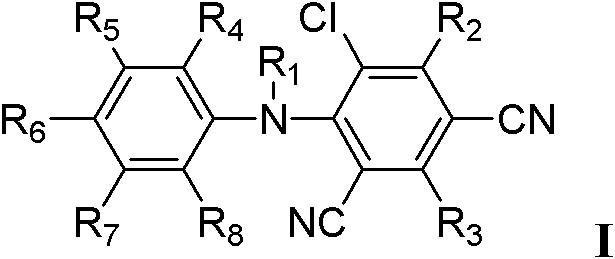
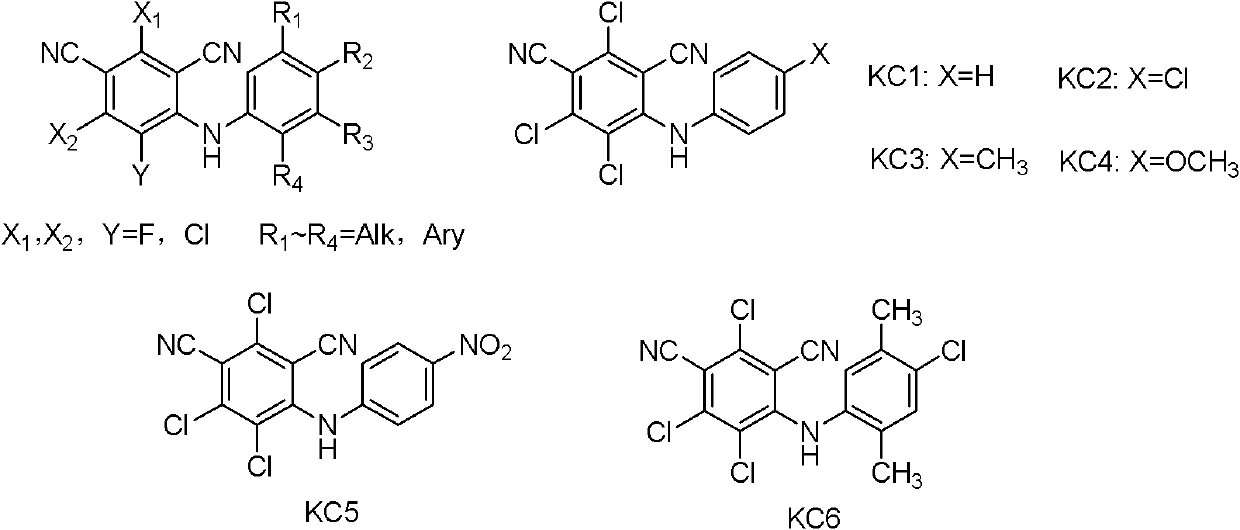
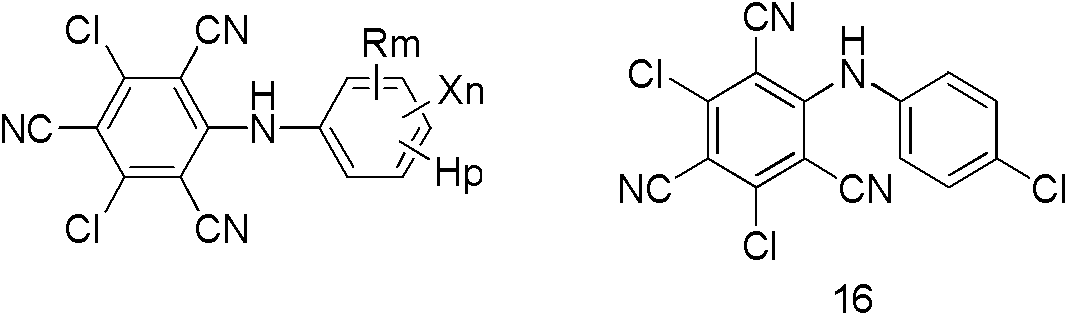
Cyano-diphenylamine-containing compounds and applications thereof
A technology containing cyanodiphenylamine and compounds, which can be used in the fields of agricultural sterilization, herbicides and insecticides, and can solve problems such as unreported structural compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0135] Example 1: Preparation of Compound 3
[0136]
[0137] To 1.03g (0.008mol) of 2,6-difluoroaniline in 40ml of N,N-dimethylformamide solution, add 0.64g (0.016mol) of sodium hydroxide, slowly add 2,4,5 while stirring, 2.13g (0.008mol) of 6-tetrachloro-1,3-benzenedicarbonitrile. After the addition, continue to stir at room temperature for 5h. After the reaction is monitored by TLC, the reaction solution is poured into water, extracted with ethyl acetate, and the organic phase is sequentially Washed with water, washed with saturated brine, dried, filtered, precipitated, and the residue was purified by column chromatography (eluent: ethyl acetate and petroleum ether (boiling range 60-90°C), volume ratio: 1:4) to obtain the product 1.65 g, namely compound 3. Pale yellow solid, melting point 264-266°C. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 ) δ (ppm): 6.70 (s, 1H, NH), 7.07 (t, 2H, Ph-3, 5-2H, J = 8.1 Hz), 7.37 (m, 1H, Ph-4-1H).
example 2
[0138] Example 2: Preparation of Compound 4
[0139]
[0140] First place 0.68g (0.002mol) of compound 3 in 20ml of concentrated sulfuric acid solution in an ice bath, slowly add the prepared mixed acid (the amounts of nitric acid and sulfuric acid are 0.004mol and 0.006mol respectively) under stirring, and control the temperature at Below 20°C. After the dropwise addition, the reaction was continued for 5 min. After the reaction was monitored by TLC, the reaction solution was poured into ice water and stirred. After cooling to room temperature, ethyl acetate was extracted, and the organic phase was successively washed with saturated brine, dried, filtered, and desolvated, and the residue was column chromatographed (eluents were ethyl acetate and petroleum ether (boiling range 60-90°C), The volume ratio is 1:4) to obtain 0.40 g of the product, namely compound 4. Light white solid, melting point 204-206°C. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ (ppm): ...
example 3
[0141] Example 3: Preparation of compound 131
[0142]
[0143] To 10.33g (0.039mol) 4-amino-3,5-dichlorobenzoic acid methyl ester (preparation method refers to WO2010060379, CN101337940) in 60ml N, N-dimethylformamide solution, add sodium hydroxide 3.12g ( 0.078mol), slowly added 2,4,5,6-tetrachloro-1,3-benzenedicarbonitrile 10.37g (0.039mol) under stirring, and continued to stir at room temperature for 5h after the addition was completed. After the reaction was monitored by TLC, The reaction solution was poured into water, extracted with ethyl acetate, the organic phase was washed successively with water, washed with saturated brine, dried, filtered, precipitated, and the residue was column chromatographed (eluent was ethyl acetate and petroleum ether (boiling range 60- 90°C), and the volume ratio was 1:5) to obtain 13.65 g of the product, namely compound 131. Pale yellow solid, melting point 229-231°C. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ (ppm): 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com