Benzoxazine resin mid-body containing arylamine and preparation method thereof
A technology of benzoxazine and aromatic amide, applied in the field of benzoxazine resin intermediates containing aromatic amide and its preparation, can solve the problems of unfavorable industrial application of benzoxazine materials, complex catalyst structure, cumbersome synthesis reaction, etc. , to achieve the effect of being suitable for industrial production, simple in structure and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
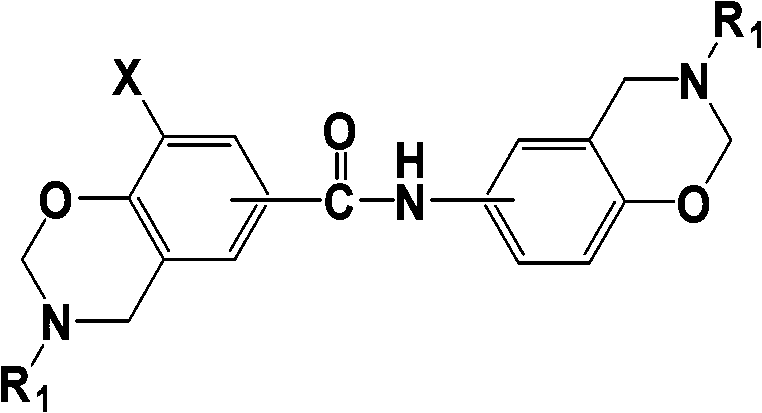
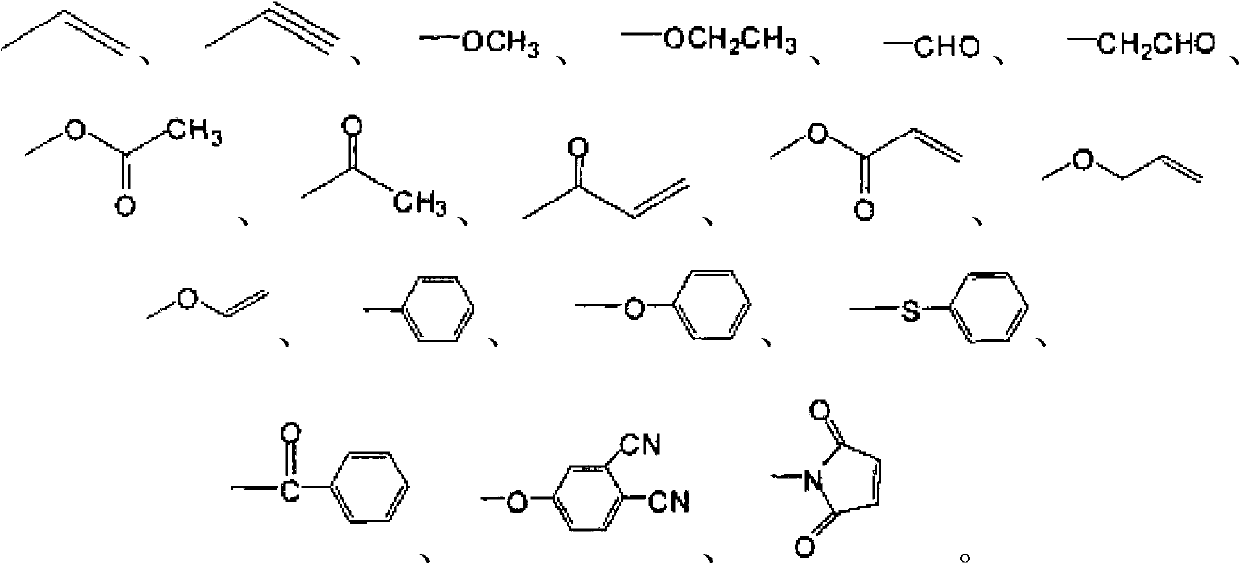
[0030] (1) The intermediate X of benzoxazine resin containing aromatic amide is -H, R 1 for
[0031] The triazine compound X is -H, R 1 for and 4,4'-dihydroxydiphenylamide with a molar ratio of 1.3 based on the triazine compound, and paraformaldehyde equimolar to the diphenol raw material are added to ethanol, and the total mass of the raw material is 60% of the volume of ethanol %, raised the temperature to 80°C and stirred for 4 hours. After stopping the reaction, washed with potassium hydroxide lye, then washed with water until neutral, filtered and dried to obtain the product with a yield of 91%. The infrared spectrum of the product is as follows:
[0032] IR(KBr,cm -1 ): 3097(Ar-H), 1660(C=O), 1508(s), 1396(C-N), 1241(C-O), 1148(C-O), 924(m).
Embodiment 2
[0034] (1) The intermediate X of benzoxazine resin containing aromatic amide is -H, R 1 for R 2 for-H
[0035] The triazine compound X is -H, R 1 for R 2 It is -H, and 3,4'-dihydroxydiphenylamide with a molar ratio of 1.5 based on the triazine compound, and paraformaldehyde equal to the mole of the diphenol raw material are added to methanol, and the total mass of the raw material is 40% of the volume of methanol, heated to 70°C and stirred for 6 hours. After stopping the reaction, washed with sodium hydroxide lye, then washed with water until neutral, filtered, and dried to obtain the product with a yield of 85%. The infrared spectrum of the product is as follows:
[0036] IR(KBr,cm -1 ): 3069(Ar-H), 1656(C=O), 1505(s), 1386(C-N), 1231(C-O), 1098(C-O), 935(m).
Embodiment 3
[0038] (1) The intermediate X of benzoxazine resin containing aromatic amide is -H, R 1 for R 2 For -CN. (cyano para substitution)
[0039] The triazine compound X is -H, R 1 for R 2 -CN. (para-substituted cyano group), and 3,3'-dihydroxydiphenylamide with a molar ratio of 1.7 based on the triazine compound, and paraformaldehyde in equimolar ratio to the diphenol raw material Into butanone, the total mass of raw materials is 30% of the volume of butanone, the temperature is raised to 80°C and the reaction is stirred for 5 hours. After the reaction is stopped, it is washed with potassium carbonate alkali solution, then washed with water until neutral, filtered and dried to obtain the product. The yield is 82%. The infrared spectrum of the product is as follows:
[0040] IR(KBr,cm -1 ): 3072(Ar-H), 1659(C=O), 1511(s), 1382(C-N), 1236(C-O), 1085(C-O), 945(m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com