Hydrogenation method for organic catalyst normal-pressure catalytic butyronitrile rubber latex
A technology for catalyzing nitrile and organic catalysts at atmospheric pressure, which is applied in the field of in-situ hydrogenation of nitrile latex at atmospheric pressure, which can solve problems such as affecting performance and difficult removal, achieve stable chemical properties, save reaction raw materials, and reduce costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
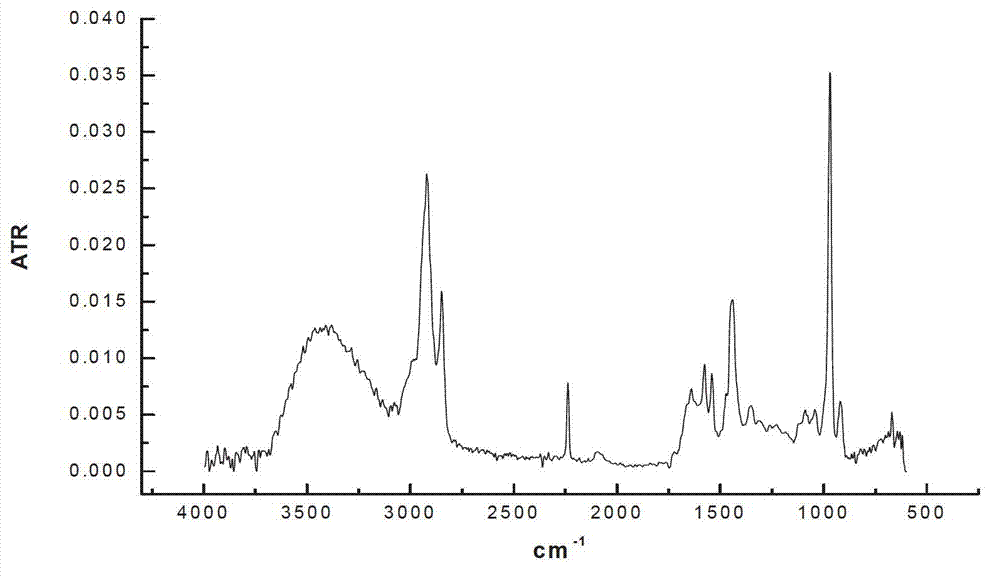
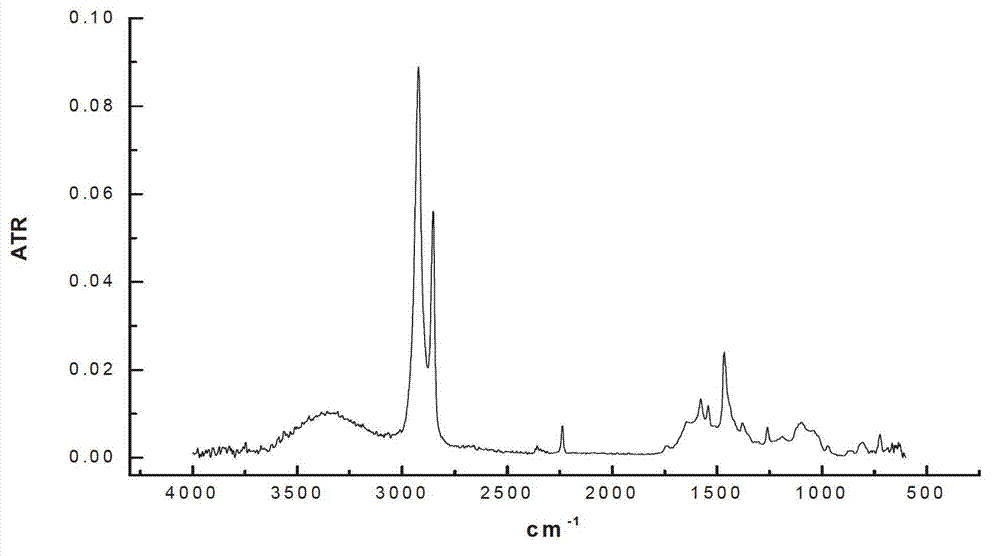
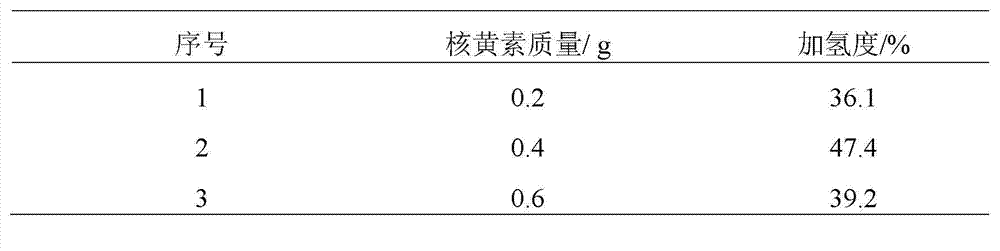
[0022] Add 100 mL of nitrile latex (34% acrylonitrile content) to a three-neck round-bottomed flask, raise the reaction temperature to 70 °C, and then add 15 mL of hydrazine hydrate (the molar ratio of hydrazine hydrate to carbon-carbon double bond is 10.0). The add-on of flavin catalyst is respectively 0.2g, 0.4g, 0.6g, under the condition of air existence, reacts 12 h, the hydrogenated nitrile latex obtained with CaCl 2 The aqueous solution was demulsified, dried, and its hydrogenation degree was calculated by measuring the infrared spectrum. The results of the hydrogenation degree are shown in Table 1.
[0023] Table 1 The effect of the amount of riboflavin catalyst added on the degree of hydrogenation
[0024]
Embodiment 2
[0026] Other conditions are the same as in Example 1, using 0.4 g of riboflavin catalyst, changing the molar ratios of hydrazine hydrate and carbon-carbon double bonds to 5.2, 10.0, 15.5, 20.6 respectively, and the hydrogenation degree results are shown in Table 2.
[0027] Table 2 Effect of the molar ratio of hydrazine hydrate to carbon-carbon double bond on the degree of hydrogenation
[0028]
Embodiment 3
[0030] Other conditions are the same as in Example 1, using 0.4 g of riboflavin catalyst, changing the reaction temperature to 50° C., 70° C., and 80° C., and the hydrogenation degree results are shown in Table 3.
[0031] Table 3 Effect of reaction temperature on degree of hydrogenation
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com