Kit and detection method for rapid quantitative detection of hepatitis virus nucleic acid
A hepatitis virus, quantitative detection technology, applied in the field of biomedical clinical diagnosis, can solve the problems of complex operation, poor accuracy, low sensitivity, etc., to achieve high sensitivity, avoid nucleic acid loss, and optimize the effect of PCR reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
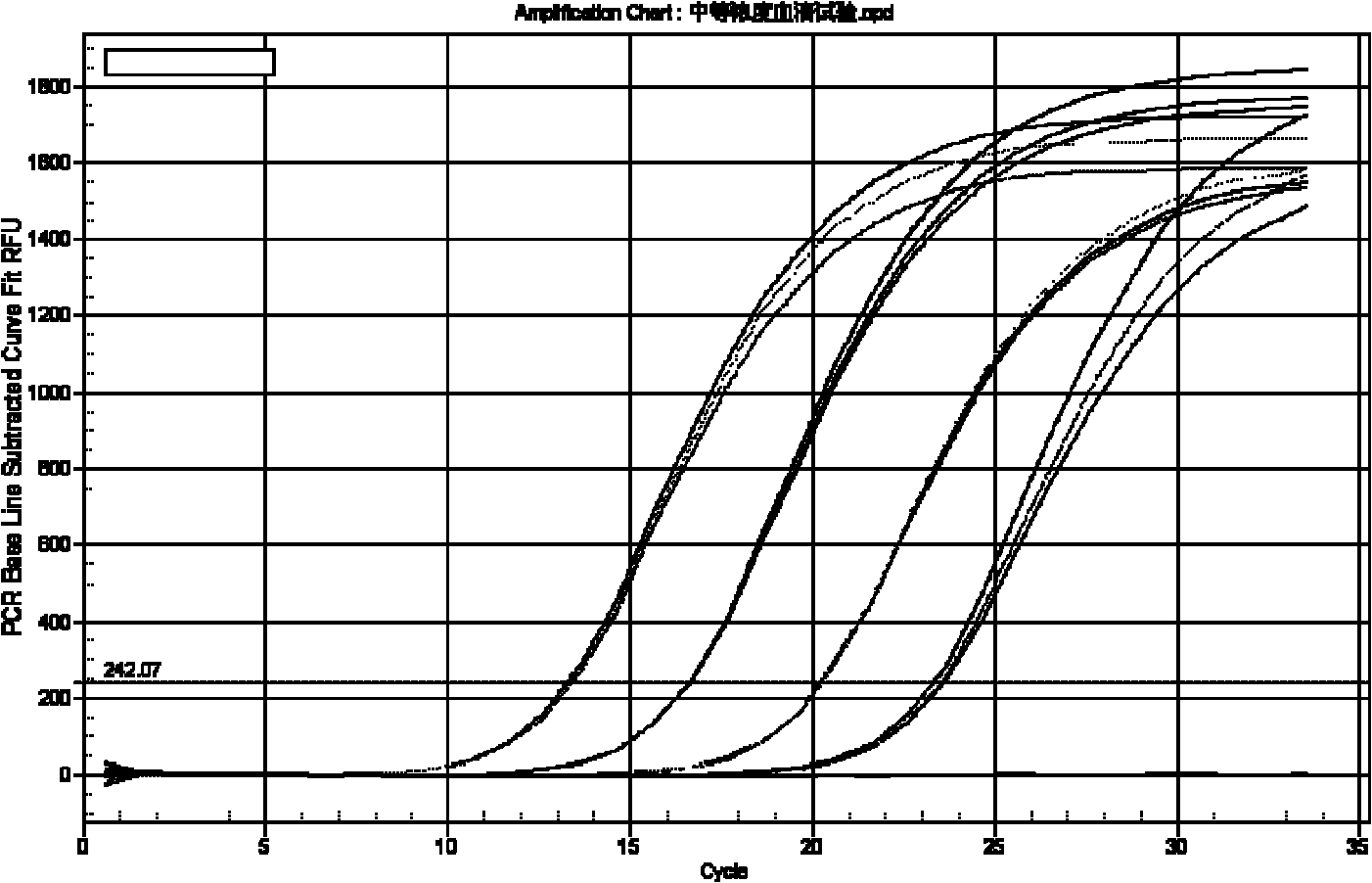
[0046] The detection of embodiment 1 HBV negative and positive serum
[0047] Take 5 μl of nucleic acid release agent (0.25% SDS, 0.25% Chaps, 5% (NH 4 ) 2 SO 4 , composed of 1% formamide. ) into the PCR reaction tube. Then draw 5 μl of serum samples to be tested, positive control, negative control, HBV DNA positive virus serum (1000IU / ml, 500IU / ml) of known concentration, join in the nucleic acid releasing agent, and pipette gently sucks for 5 -8 times, rest at room temperature for 10 minutes. Then add 1 μl internal standard (known positive internal control-500copies / μl) to each tube according to the number of reactions, and finally add 40 μl of the prepared PCR reaction solution. The composition of the reaction solution is as follows, and perform fluorescence quantitative PCR on Bio-Rad IQ5 Amplification, the procedure is as follows.
[0048] PCR reaction solution components
[0049] Element
Dosage
10×Buffer
5.0μl
dNTPS
5.0μl
...
Embodiment 2
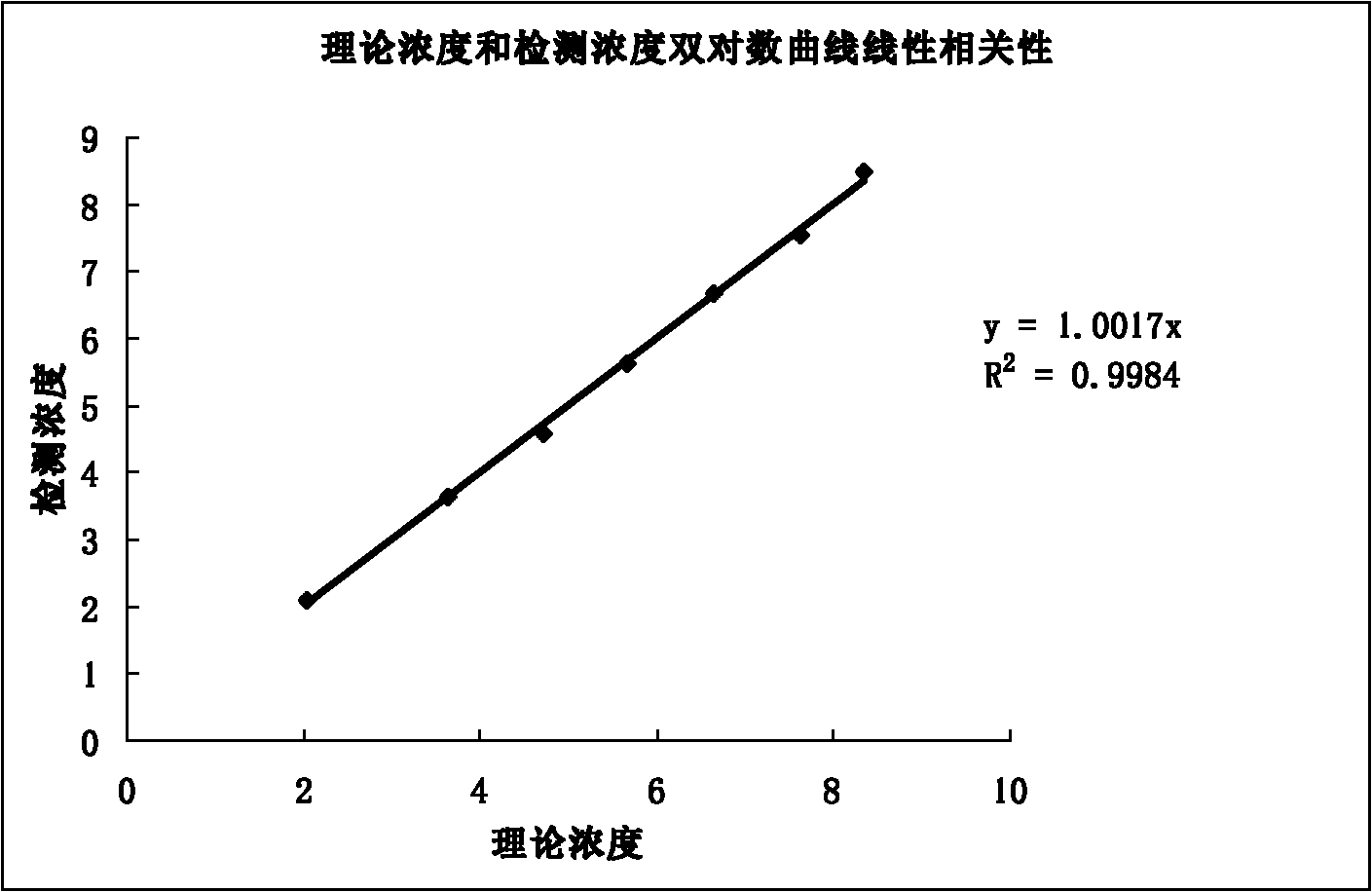
[0058] Embodiment 2. comparative analysis of the present invention and the kits of mainstream manufacturers
[0059] Use this kit and kits from domestic mainstream manufacturers to detect 3 HBV DNA positive sera at the same time. The operating method of the present invention is the same as above. The specific process of kits from mainstream manufacturers is as follows: take 100 μl of the sample to be tested, the positive control, the negative control, and 3 parts of HBV DNA positive serum with a filter tip, and add them to the centrifuge tube, then add 100 μl of sample treatment solution A, shake and mix. uniform. Centrifuge at 12000 rpm for 10 minutes, then discard the supernatant, add 25 μl of sample treatment solution B to the pellet, and pipette 8-10 times. Boil the centrifuge tube in boiling water at 100°C for 10 minutes, then take out the centrifuge tube and centrifuge at 12,000 rpm for 10 minutes, and finally take 2 microliters of the supernatant for PCR amplification...
Embodiment 3
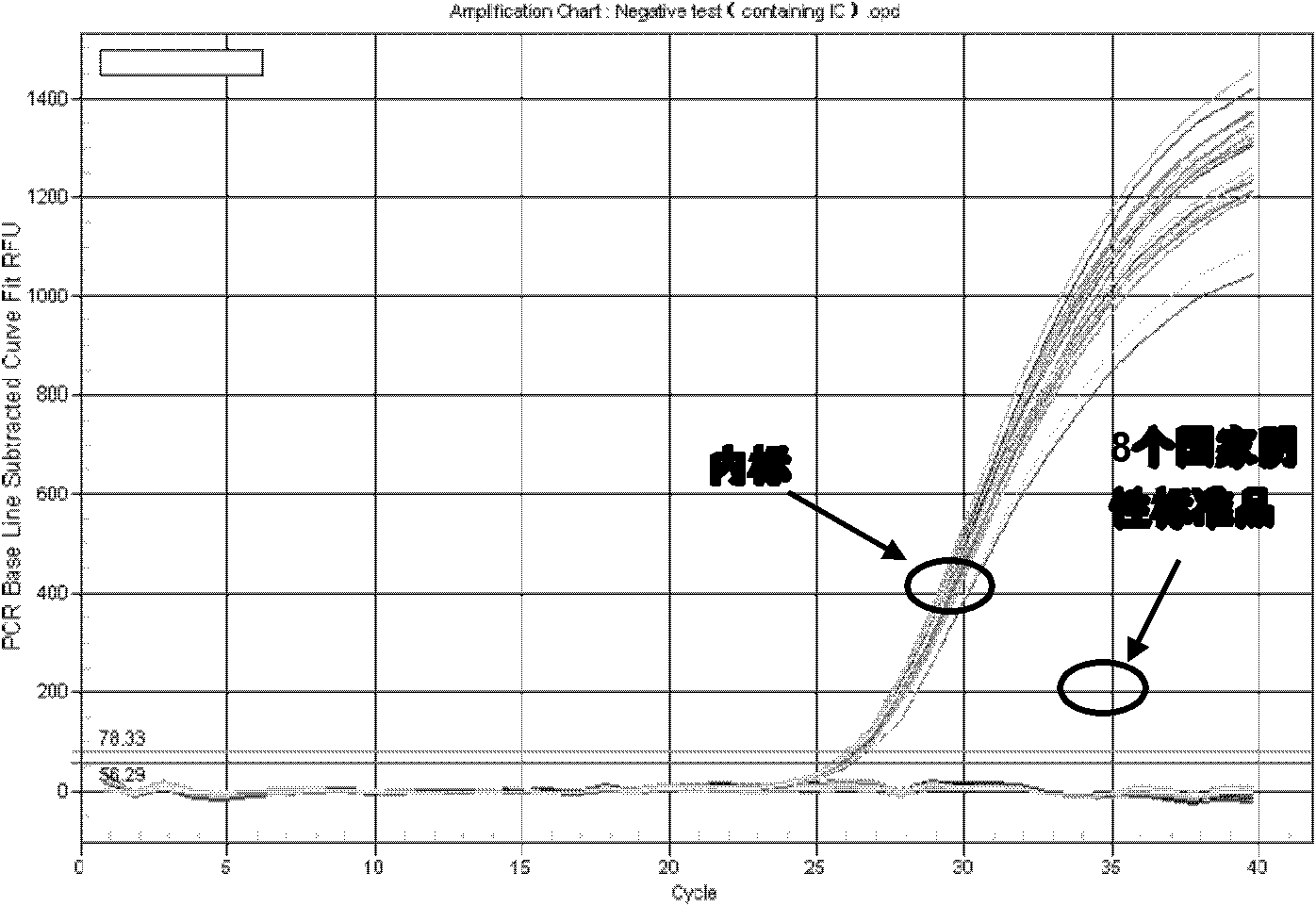
[0063] Example 3 Rapid detection of HCV clinical samples
[0064] Take 5 μl of nucleic acid release agent (0.25% SDS, 0.25% Chaps, 5% (NH4) 2 SO 4 , composed of 1% formamide. ) into the PCR reaction tube. Then take 5 μl of the serum samples to be tested, positive control, negative control and 4 copies of HCV RNA positive virus serum of known concentration, add them to the nucleic acid release agent, pipette gently for 5-8 times, and stand at room temperature for 10 minute. Then add 1 μl internal standard (known positive internal control-500copies / μl) to each tube according to the number of reactions, and finally add 40 μl of the prepared PCR reaction solution. The composition of the reaction solution is as follows, and perform fluorescence quantitative PCR on Bio-Rad IQ5 Amplification, the amplification procedure is as follows.
[0065] PCR reaction solution components
[0066] Element
Dosage
10×Buffer
5.0μl
dNTPS
5.0μl
HotStar ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com