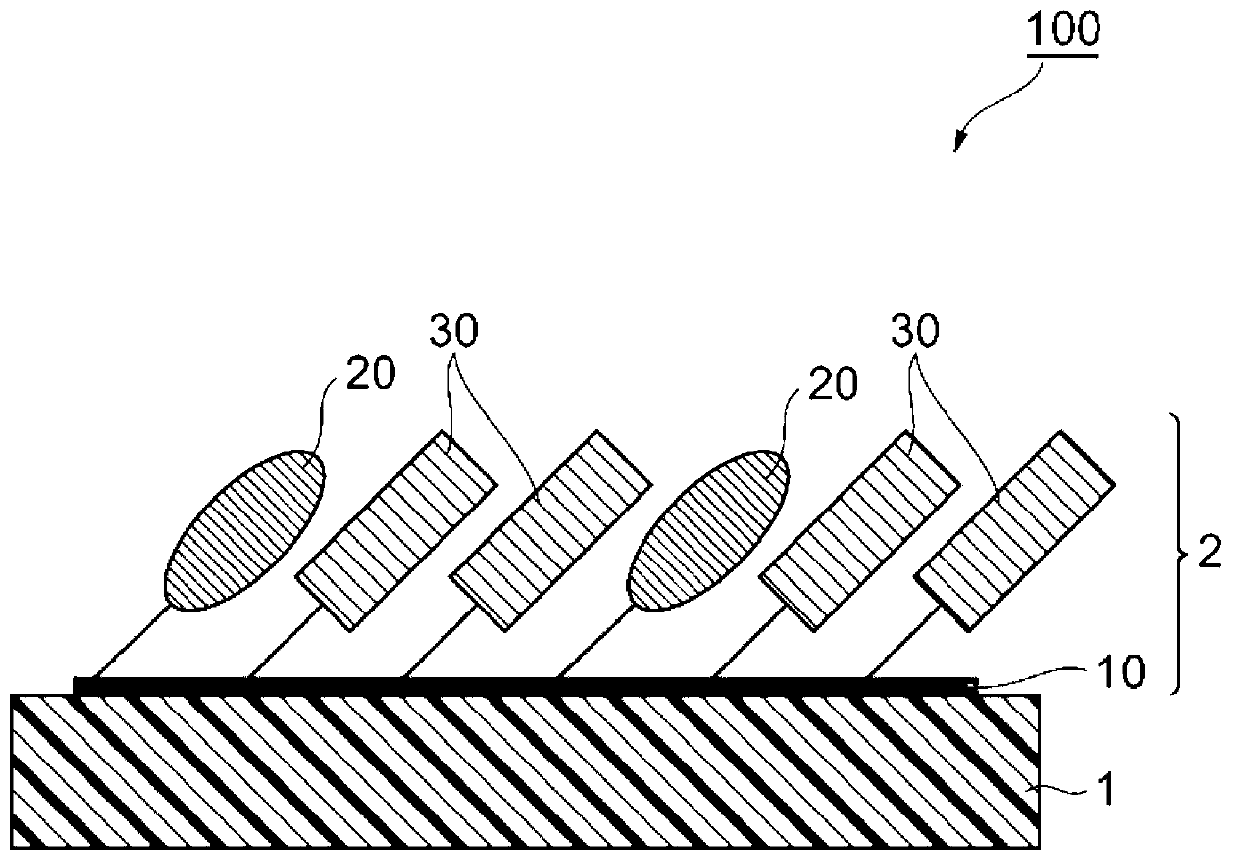
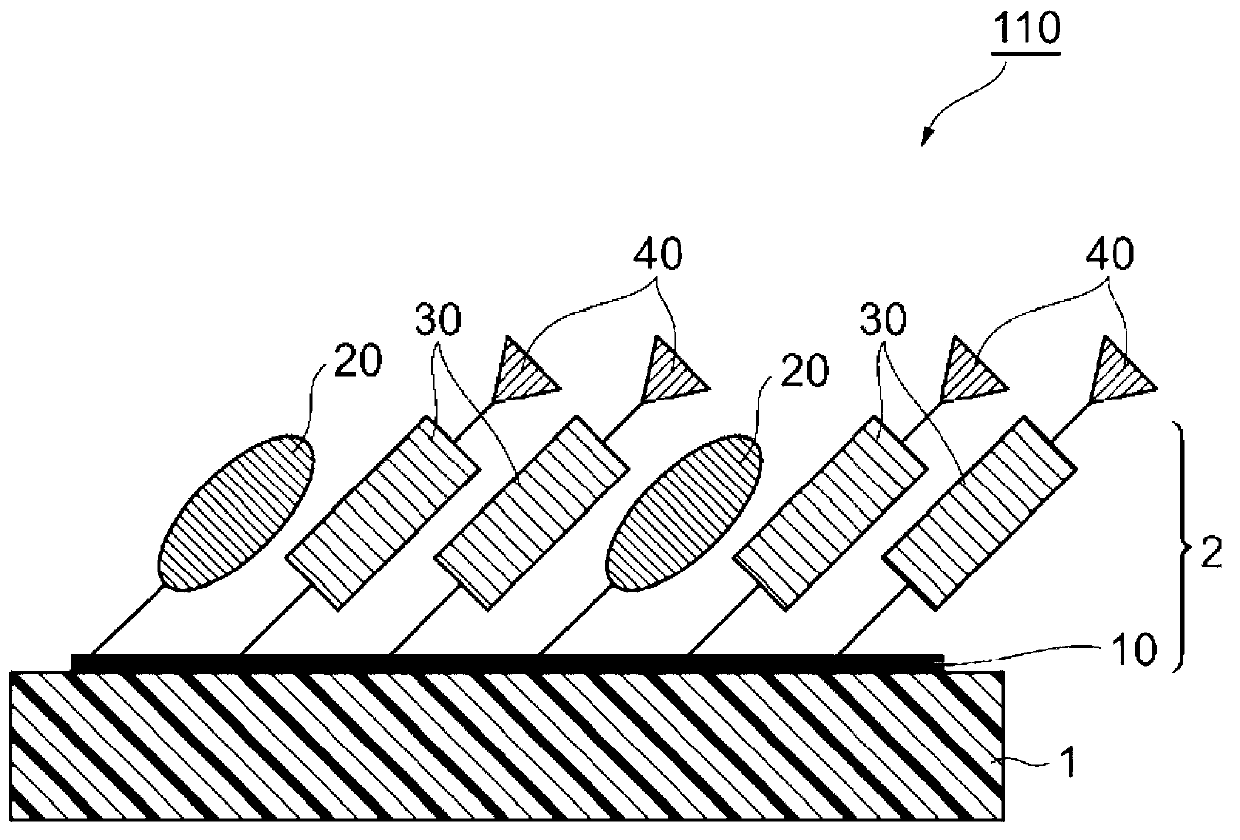
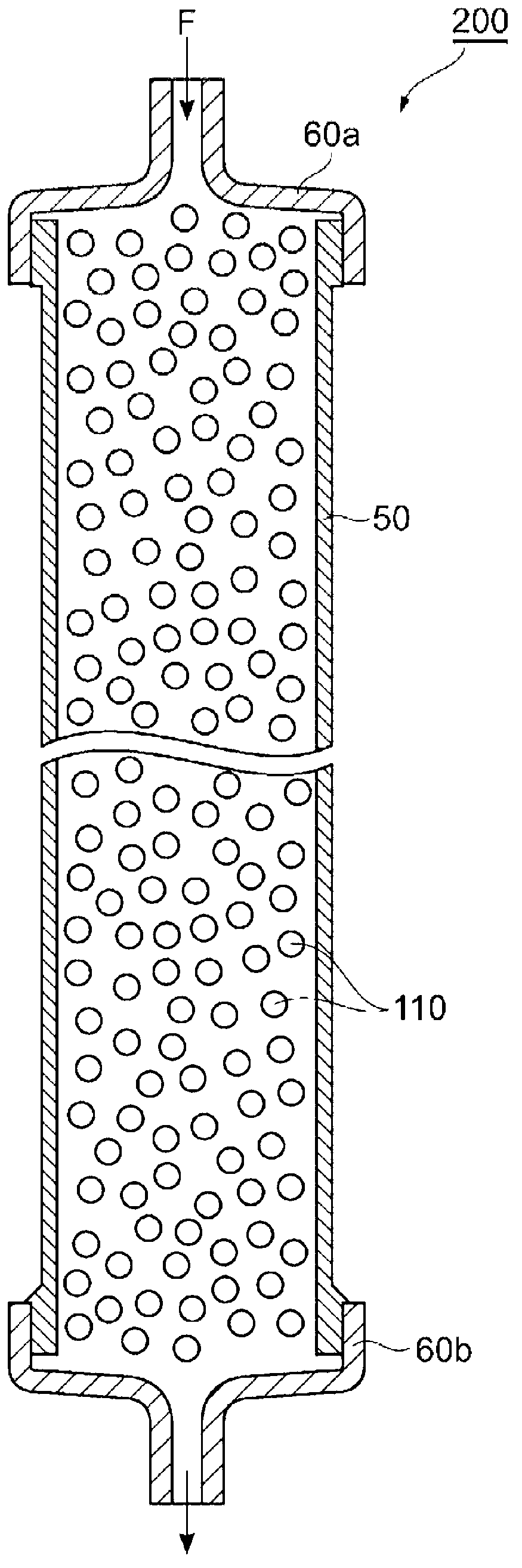
Substrate for ligand immobilization and method for producing same
A manufacturing method and technology of ligands, applied in chemical instruments and methods, other chemical processes, blood diseases, etc., can solve problems such as small interactions, and achieve the effect of high adsorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] (A) method
[0094] (1) Gamma ray irradiation
[0095] A non-woven fabric made of polypropylene (average fiber diameter 3.8 μm, weight per unit area 80 g / m) was used as a carrier 2 )0.108m 2 Seal it together with a deoxidizer in a low-oxygen-permeable bag, and after fully removing oxygen, irradiate with 25kGy of γ-rays at -78°C.
[0096] (2) Grafting reaction
[0097] Dissolve 10.8 g of N-methacryloyloxyethyl-N,N-dimethylammonium-α-N-methylcarboxybetaine and 26.3 mL of glycidyl methacrylate (GMA) in 500 mL of methanol at 40 °C for 60 minutes with nitrogen.
[0098] The above-mentioned non-woven fabric was quickly put into a pressure-resistant glass container, and after decompression, the above-mentioned solution was introduced and allowed to react at 40° C. for 1 hour. After the reaction, the taken out nonwoven fabric was washed with dimethylformamide and methanol, and vacuum-dried at 40° C. to obtain a nonwoven fabric for ligand immobilization (substrate for ligan...
Embodiment 2
[0126] Dissolve 21.5g of N-methacryloyloxyethyl-N,N-dimethylammonium-α-N-methylcarboxybetaine and 26.3mL of glycidyl methacrylate in 500mL in the same manner as in Example 1 The reaction solution was prepared in methanol, and the grafting reaction was carried out to obtain a non-woven fabric for ligand immobilization. As a result of surface analysis by XPS, the molar composition ratio R of CMB was 0.26, and the coverage S was 54%. In addition, the measured specific surface area was 0.50 m 2 / g. A cell adsorption filter was fabricated from this nonwoven fabric, and the amount of antibody immobilized was measured. As a result, 11.6 mg of antibody was immobilized on the cell adsorption filter before washing, and 8.4 mg of antibody was immobilized on the cell adsorption filter after SDS washing. As a result of producing a cell adsorber from this cell adsorption filter and measuring the adsorption rate of cells, the adsorption rate of CD4-positive cells was 99.7% (F1) and 89.0% (...
Embodiment 3
[0128] Dissolve 43.1g of N-methacryloyloxyethyl-N,N-dimethylammonium-α-N-methylcarboxybetaine and 26.3mL of glycidyl methacrylate in 500mL of The reaction solution was prepared in methanol, and the grafting reaction was carried out to obtain a non-woven fabric for ligand immobilization. Based on the results of surface analysis by XP S, the molar composition ratio R of CMB was 0.32, and the coverage S was 54%. In addition, the measured specific surface area was 0.51 m 2 / g. A cell adsorption filter was fabricated from this nonwoven fabric, and the amount of antibody immobilized was measured. As a result, 9.6 mg of antibody was immobilized on the cell adsorption filter before washing, and 8.8 mg of antibody was immobilized on the cell adsorption filter after SDS washing. As a result of producing a cell adsorber from this cell adsorption filter and measuring the adsorption rate of cells, the adsorption rate of CD4-positive cells was 95.1% (F1) and 86.0% (F2). In addition, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com